Abstract
We investigated age-related changes in the styloid process in 88 skulls, aged from 5 months to 85 years of age. The osseous styloid process was not well developed in children. Its length increased significantly with age (from 2.3 mm in 11–20 age group to 16.3 mm in 61–85 group). In adolescent specimens (11–20 years of age), the median distance from the styloid process to the stylomastoid foramen was 0.7–0.8 mm, whereas in adult and old age specimens the two structures were completely adjacent or very close, usually less than 0.2 mm. The process was missing in 5% of the adult specimens. There was a statistically significant positive association between the length of the styloid process with age (r = 0.3210, 95% CI 0.0817–0.5254; P = 0.0097), whereas the distance from the styloid process to the stylomastoid foramen significantly decreased with age (r = −0.4518, 95% CI −0.6167 to −0.2490; P = 0.0001). Changes in the length and shape of the styloid process reflected altered function of the three muscles originating from the styloid process—m. stylopharyngeus, m. stylohyoideus and m. styloglossus. They have a common function of lifting the aerodigestive elements upward and backward, after the descent of the aerodigestive tract and final morphological differentiation of the vocal system during puberty. Relationship between altered muscle function and the morphology of the styloid process are important for understanding the clinical syndromes related to the styloid process, such as Eagle’s syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The styloid process is a part of the stylohyoid complex which includes bony and ligamentous structures: styloid process, cornu minus of the hyoid bone, stylohyoid ligament, and stylomandibular ligament, all embryological derivatives of the second branchial arch [5]. Styloid processes may be asymmetrical, varying in lengths from 0.1 to 4.2 cm, and missing in 58% of the cases [1]. The styloid process is surrounded by a bony cuff called the sheath of the styloid process, vagina processus styloidei. The vagina is the product of the tympanic cavity medially and of the tympanic part of the temporal bone laterally [11, 18]. The styloid process starts to ossify from two centers: one in the proximal part of the hyoid arch (tympanohyal part), which appears before birth, and the other in the distal part of the hyoid arch (stylohyal part), which appears in the second half of the first year of life [13, 15]. Full ossification of the styloid process occurs between the fifth and eighth year of life [2, 7]. The stylohyoid complex is rarely completely ossified [3, 4, 10, 16]. Excessive ossification of the stylohyoid complex may lengthen the styloid process and thus compromise nearby vascular and neural structures, especially the glossopharyngeal nerve [2]. Eagle’s syndrome [6] is the clinical presentation of elongated styloid process and includes recurrent craniofacial and cervical pain, difficulties in swallowing, throat pain and foreign body sensation, and secondary glossopharyngeal neuralgia with pain radiating into the maxillar and orbital regions. Eagle’s syndrome occurs in up to 27% of the population [16].
One of the reasons for excessive ossification and angulation of the styloid process in man may be related to the function of the muscles originating from the styloid process in the phonation and deglutition. The three muscles originating from the styloid process have a common function in adults—lifting the aerodigestive elements upward and backward. Despite identical function, the three muscles—m. stylopharyngeus, m. stylohyoideus, and m. styloglossus, the so called bunch of Riolanus—have different innervation (n. IX, n. VII, and n. XII, respectively) and destination. Although they are involved in phonation, the changes in their morphology related to this ontogenically newly acquired function are not so well described as for other muscles involved in these processes. For example, m. levator veli palatini has a nearly horizontal position in the newborn [15], and then moves vertically with the descent of the throat organs. Also, the change in the function of the muscles related to the naso- and oropharynx influences the morphology of the pterygoid hamulus [8] and the whole epipharygeal region of the cranial base [9]. There are few systematic studies of the changes in the morphology of the styloid process and related osseous structures of the temporal bone, especially in regard to the changes occurring during the postnatal change in the aerodigestive tract. We analyzed the changes in the morphology of the styloid process and its osseous sheath, as well as relation to the stylomastoid foramen in 88 skull specimens aged from 5 months to 85 years.
Materials and methods
We investigated the styloid process and the vagina p. styloidei on 27 macerated, disarticulated skulls from 5 months to 20 years of age and 61 macerated skulls from 21 to 85 years of age. The skulls are a part of the historical Zagreb skull collection [8, 9].
We recorded the presence of the process and measured its length from the attachment to the bone to the apex, the symmetry of the processes on both sides of the skull, and relation to the stylomastoid foramen.
The measurements were grouped into four age groups: postnatal period until the end of process ossification (5 months to 9 years), adolescence (11–20 years), adult age (21–60 years), and old age (62–85 years). The data for age groups were tested for normality of distribution, and presented as mean ± standard deviation for normally distributed data, or median and range for non-normally distributed data. Data were compared by Student t test for normally distributed data and Mann–Whitney test for non-normally distributed data. The association between age and anatomical measurements was tested by the correlation coefficient, and presented graphically as a dot-plot with a regression line.
Results
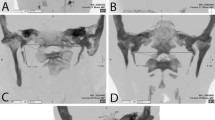
As the styloid process fully ossifies after 8 years of age [2, 7], we could not statistically analyze the data on 12 skulls aged from 5 months to 9 years (5 female and 7 male specimens). The most prominent morphological changes during the development of the styloid process and related structures in these 12 specimens are presented in Fig. 1. In youngest specimens the osseous styloid process was either absent or very small, hidden within a groove bordered laterally by the prolongation of the pars tympanica of the temporal bone, and medially by the bone of the pyramid. The length of this prolongation of the pars tympanica increased up to the age of 9 years, when the osseous styloid process was well developed, and the vagina p. styloidei measuring up to 5 mm on both sides. The average distance of the styloid from the stylomastoid foramen was 2 mm. The pyramidal part of the sheath of the styloid process disappeared gradually due to the increasing mass of the styloid process. With the fusion of the base of the styloid process with the pyramid of the temporal bone, the styloid process approached the stylomastoid foramen, so that in the adult specimens the opening of the facial canal was often in direct contact with the styloid process (Fig. 2). In adolescent specimens (11–20 years of age), the median distance from the styloid process to the stylomastoid foramen was 0.7–0.8 mm, whereas in adult and old age specimens the two structures were completely adjacent or very close, usually less than 0.2 mm (Table 1).
Morphology of the styloid process, vagina processus styloidei and their relationship with the foramen stylomastoideum: temporal bones from a 6-month-old male, 2-year-old male, 7-year-old female and 9-year-old male. Arrows indicate the ossified parts of the styloid process in the vagina p. styloidei. The tympanale develops earlier, in fetal life, but is lost in most specimens because of the maceration process; it is found only in specimens where it is fixed to the pyramid. MP mastoid process, CC carotid canal, SF stylomastoid foramen
Morphology of the styloid process and vagina p. styloidei and their relationship with the foramen stylomastoideum (arrow) in adults (left image: 21-year-old male, middle image: 78-year-old female, right image: 63-year-old female). In young adults, the vagina p. styloidei is clearly separated from the styloid process (left image). In old individuals, the processus styloideus and its vagina are often fused and provide massive base for the styloid process and attached muscles. The styloid process is close to the stylomastoid foramen, separated either by a thin bony plate (middle image) or being full incorporated in its anterior wall (right image)
In adult specimens, the process was of different shapes and of variable length, from 3 to 40 mm (Table 1). There was no difference in the measurements on the left and the right side of the skull (P range 0.2245–0.8202 for different age groups). There were no statistical differences between male and female specimens (P range 0.3456–0.9564).
In old age, the tympanic part of the vaginal process developed a closer contact with the styloid process, enveloping its base from the lateral side so that it was sometimes impossible to determine the limit between the two structures. In some cases, the vagina p. styloidei disappeared completely, i.e., fused with the base of the styloid process (Fig. 2). When the proximal part of the styloid process was well developed, it was massive but became slender toward its ending point. The styloid process was missing in adults in 3 (5%) out of 61 examined cases.
There was a statistically significant positive association between the length of the styloid process with age (r = 0.3210, 95% CI 0.0817–0.5254; P = 0.0097), whereas the distance from the styloid process to the stylomastoid foramen significantly decreased with age (r = −0.4518, 95% CI −0.6167 to −0.2490; P = 0.0001) (Fig. 3). Age had no influence on the length of the vagina processus styloidei (r = 0.2157, 95% CI −0.0239 to 0.4319; P = 0.0773).
Discussion
Our study showed that the most intensive changes in the morphology of the styloid process, especially in relation to the vagina p. styloidei and the surrounding structures, occurred during the early postnatal period, up to 9 years of age. Prolongation of the styloid process, i.e., its full ossification was most intensive in the adolescent years, with only a small increase in length after 20 years of age until old age. With the increasing age in adults, the distance between the styloid process and the foramen stylomastoideum decreased, and very often completely disappeared. Figure 4 summarizes major structural changes and relationships of the styloid process and related structures of the temporal bone during postnatal period.
Schematic presentation of the pyramid base with the styloid process and stylomastoid foramen. In a 1-year-old child (left image; a), the ossified styloid process (black circle; SP) is deep in the basis of the pyramid, at a distance from the stylomastoid foramen (open circle; SF). In young adolescents (middle image, b), the ossified styloid process is longer and the posterior part of the tympanic ring forms the vagina p. styoidei. In adults (right image, c), the styloid process is fully ossified and is long and massive, enveloped by the vagina p. styloidei. The distance between the styloid process and stylomastoid foramen is very small or absent
The occurrence of most intensive changes in the morphology of the styloid complex and anatomical relation with the developing surrounding structures, observed in our study in specimens up to 9 years of age, correspond to the greatest changes in the position of the hyoid bone and the larynx in relation to the palate and mandible, unique for humans [12]. The time after birth up to the age of 8 years is the time of hyolaryngeal descent, which is influenced both by spatial constraints related to deglutition and vocalization. This is the time when the ratio of pharynx height to the length of the oral cavity decreases from 1.5 at birth to 1.0—optimal ratio according to the quantal theory of speech [12]. At the same time, the descent of the hyoid and larynx relative to the mandible is restricted by the function of the muscles involved in deglutition [12] because the coordination of the suprahyoid and infrahyoid muscles is at least partly a function of the spatial relationships between the structures involved in swallowing—the mouth, mandible, hyoid, and epiglottis.
The styloid muscles originate at the base of the styloid process and the vaginal process and have different destinations [1, 13, 15]. When the position and the morphology of the styloid processes in formative postnatal age and in adults are taken into account, the function of the styloid muscles differs greatly in young children during the most intensive hyolaryngeal descent up to the age of 8 years compared with that in adults. The differences in the position of the styloid process and styloid muscles and their relation to the cranial base and the descending pharynx are schematically presented in Fig. 5. In children, the position of the muscles originating from the styloid process is nearly horizontal and the main result of muscle function is widening of the aerodigestive tract. M. stylopharyngeus has greatest importance in this respect as its position in relation to the pharynx in an infant results in widening of the pharynx during sucking, producing a certain degree of under-pressure, thus facilitating the movement of the bolus or fluid from the mouth to the pharynx.
Only after the full descent of the aerodigestive tract, the course of styloid muscles becomes more vertical, and the movement resulting from their contraction lifts and pulls the structures of the aerodigestive tract upward and backward, both in swallowing and phonation. M. styloglossus enters the tongue and intermingles with the longitudinal tongue muscles [15, 17]. It is innervated by the hypoglossal nerve, like the other muscles of the tongue; its final, adult function of pulling the tongue posteriorly is present immediately after birth and does not change. M. stylohyoideus inserts on the lesser horn of the hyoid bone and is innervated by the facial nerve—the nerve of the second branchial arch. The muscle is in full function only in adults, after the descent of the aerodigestive tract. M. stylopharygeus enters the pharyngeal tube between the superior and middle constrictor and can be followed as far as the thyroid or even cricoid cartilage. This muscle realizes its full function, widening and lifting of the middle part of the pharyngeal tube, only after the complete descent of the aerodigestive tract. The described changes in the function of the muscles attached to the styloid influences its final size and shape. As the styloid process is a part of the styloid complex, composed of several bony parts connected by connective tissue of the stylohyoid ligament, the ossification of the structures along the styloid complex contributes to the length of the ossified styloid process and its relation to the surrounding structures. A very long styloid process can cause glossopharyngeal neuralgia, so that clinically or radiologically unrecognized elongation of the styloid results in unnecessary neurosurgical intervention [19].
Most constant is the most proximal part of the styloid complex, the tympanale, which becomes fixed to the temporal pyramid in the second half of the first year of life. The medial part of the tympanale leans against the pyramid, while its lateral part is covered by a triangular prolongation of the tympanic bone forming the vagina p. styloidei. In our series, the tympanale in children generally remained at the level of the inferior surface of the pyramid, while in adults it was of variable length in cases when it was present. The contact of the vagina p. styloidei and the styloid process became in adults so close that it was sometimes impossible to differentiate between the two structures. The fusion of the structures into a massive osseous base was related to the function of the styloid muscles, in a similar way as the development of the mastoid process of the temporal bone after birth in relation to the activity of the sternocleidomastoid muscle [20]. With the incorporation of the pyramidal part in the base of the styloid process, the styloid process gained close contact with the stylomastoid foramen and the facial nerve.
Although there are many studies on both the normal and abnormal morphology of the styloid process and other structures of the styloid complex in large series of adult individuals [2, 4, 6, 10, 14], there was little comprehensive information on the changes in the morphology and spatial relationships of the styloid process during ontogeny. In order to understand the complex relationships between different anatomical structures in determining the final shape of osseous landmarks of the styloid complex, especially related to deglutition and vocalization, it is necessary to study styloid morphology from postnatal to adult and old age. Our study demonstrated that the ossification of the styloid process occurs long into adult life. The underlying mechanisms for this life-long process is not clear, but may involve the variability in the length and shape of the second branchial arch cartilage [17], which can explain the cases of styloid complex ossification in adults [2, 3, 6]. It is also possible that the ossification of the branchial cartilages is a part of the normal aging process as it occurs also in other derivatives of the branchial arches, such as the laryngeal cartilages [14].
References
Anson BJ, Donaldson JA (1973) Surgical anatomy of the temporal bone and ear, 2nd edn. Saunders, Philadelphia
Bafageeh SA (2000) Eagle’s syndrome: classic and carotid artery types. J Otolaryngol 29:88–94
Baki Yagci A, Kiroglu Y, Lzdemir B, Kara CO (2008) Three-dimensional computed tomography of a complete stylohyoid ossification with articulation. Surg Radiol Anat 30:167–169
Basekim CC, Mutlu H, Gungor A, Silit E, Pekkafali Z, Kutlay M, Colak A, Ozturk E, Kzilkaya E (2005) Evaluation of styloid process by three dimensional computed tomography. Eur Radiol 15:134–139
Corning HK (1928) Lehrbuch der Entwicklungsgeschichte des Menschen, 2nd edn. Bergmann, München
Eagle WW (1962) The symptoms, diagnosis and treatment of the elongated styloid process. Am Surg 28:1–5
Gozil R, Yener N, Calguner E, Arac M, Tunc E, Bahcecioglu M (2001) Morphological characteristics of styloid process evaluated by computerized axial tomography. Ann Anat 183:527–535
Krmpotić-Nemanić J, Vinter I, Marušić A (2006) Relations of the pterygoid hamulus and hard palate in children and adults: anatomical implications for the function of the soft palate. Ann Anat 188:69–74
Krmpotić-Nemanić J, Vinter I, Ehrenfreund T, Marušić A (2006) Age-related changes in the anatomical landmarks of the osseous epipharynx. Ann Anat 188:459–467
Kurosglu P, Unalan F, Erdem T (2005) Radiological evaluation of the styloid process in young adults resident in Turkey’s Yeditepe University faculty of dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100:491–494
Le Double AF (1903) Traité des variations des os du crâne de l’homme. Vigot, Paris
Lieberman DE, McCarthy RC, Hiiemae KM, Palmer JB (2001) Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Biol 46:117–128
Martin R, Saller K (1962) III Bd. Lehrbuch der Anthropologie. G. Fischer, Stuttgart
Mupparapu M, Vuppalapati A (2005) Ossification of laryngeal cartilages on lateral cephalometric radiographs. Angle Orthod 75:196–201
Peter K, Wetzel G, Heiderich F (1934) Handbuch der Anatomie des Kindes. I Bd. J. Bergmann, München
Rizzatti-Barbosa C, Ribeiro MC, Silva-Concilio LR, Di Hipolito O, Ambrosano GM (2005) Is an elongated styloid process prevalent in the elderly? A radiographic study in a Brazilian population. Gerodontology 22:112–115
Rodríguez-Vázquez JF, Mérida-Velasco JR, Verdugo-López S, Sánchez-Montesinos I, Mérida-Velasco JA (2006) Morphogenesis of the second pharyngeal arch cartilage (Reichert’s cartilage) in human embryos. J Anat 208:179–189
Sieglbauer F (1927) Lehrbuch der normalen Anatomie des Menschen. Urban und Schwarzenberg, Berlin
Soh KBK (1999) The glossopharyngeal nerve, glossopharyngeal neuralgia and the Eagle’s syndrome—current concepts and management. Singapore Med J 40:659–665
Weiglein AH (1996) Postnatal development of the facial canal. An investigation based on cadaver dissections and computed tomography. Surg Radiol Anat 18:115–123
Acknowledgments
The research presented in the article was supported by grants from the Croatian Ministry of Science, Education and Sports (No. 108-0650445-0271 and No. 108-1080229-0140).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krmpotić Nemanić, J., Vinter, I., Ehrenfreund, T. et al. Postnatal changes in the styloid process, vagina processus styloidei, and stylomastoid foramen in relation to the function of muscles originating from the styloid process. Surg Radiol Anat 31, 343–348 (2009). https://doi.org/10.1007/s00276-008-0450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-008-0450-2