Abstract
Background
Currently, multidetector computed tomographic (MDCT) angiography has become a noninvasive alternative imaging modality to catheter renal angiography for the evaluation of renal vascular anatomy in living renal donors. In this study, we investigated the diagnostic accuracy of 16-slice MDCT in the preoperative assessment of living renal donors.
Methods
Fifty-nine consecutive living renal donors (32 men, 27 women) underwent MDCT angiography followed by open donor nephrectomy. All MDCT studies were performed by using a 16-slice MDCT scanner with the same protocol consisting of arterial and nephrographic phases followed by conventional abdominal radiography. The MDCT images were assessed retrospectively for the number and branching pattern of the renal arteries and for the number and presence of major or minor variants of the renal veins. The results were compared with open surgical results.
Results
The sensitivity and specificity of MDCT for the detection of anatomic variants of renal arteries including the accessory arteries (n = 9), early arterial branching (n = 7) and major renal venous anomalies including the accessory renal veins (n = 3), late venous confluence (n = 4), circumaortic (n = 2) or retroaortic (n = 3) left renal veins were 100%. However, the sensitivity for identification of minor venous variants was 79%. All of three ureteral duplications were correctly identified at excretory phase conventional abdominal radiography.
Conclusion
Sixteen-slice MDCT is highly accurate for the identification of anatomic variants of renal arteries and veins. Dual-phase MDCT angiography including arterial and nephrographic phases followed by conventional abdominal radiography enables complete assessment of renal donors without significant increase of radiation dose. However, the evaluation of minor venous variants may be problematic because of their small diameters and poor opacification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Living donor renal transplantation has become major treatment for patients with end-stage renal disease because of safe surgical procedure with excellent graft survival rates [11]. Comprehensive preoperative evaluation of potential renal donors is crucial for selecting proper donors and the surgical technique. In addition to assessing the renal artery, anatomic definition of the renal venous system and the status of the donor kidney and collecting system are important. Traditionally, living renal donors have undergone preoperative evaluation with excretory urography and renal catheter angiography. Renal catheter angiography was performed to assess the number of renal arteries, prehilar branching, and any vascular disease. However, it is an invasive procedure and has limited value in detailed assessment of renal venous anomalies [2]. Spiral computed tomographic (CT) angiography initially has become an accepted imaging modality for preoperative evaluation of potential renal donors [12, 17, 20]. More recently, multidetector computed tomographic (MDCT) angiography is a noninvasive technique, replacing catheter angiography and excretory urography for preoperative evaluation of potential renal donors. The advent of MDCT scanners have provided shorter image acquisition time, narrower collimation, improved temporal and spatial resolution, decreased motion and partial volume artifacts, and near isotropic data acquisition compared with single-detector spiral CT [19]. The number, size, branching pattern, course, and relationship of the renal arteries and veins are easily demonstrated by MDCT angiography [3, 5, 6, 8, 14–16, 18]. Preoperative knowledge of minor venous variants such as a prominent lumbar or gonadal vein, may facilitate the dissection of these veins and help avoid hemorrhagic complications in surgery [7]. In the most of the prior studies, only major venous variants were evaluated, whereas the minor venous ones have not been studied extensively.
In the present study, we investigated the diagnostic accuracy of 16-slice MDCT angiography in the evaluation of arterial and venous variants including minor venous variants in potential living renal donors by comparing it with the surgical results.
Materials and methods
Subjects
In this retrospective study, we included 59 living renal donors (32 men, 27 women; mean age, 42.6 years; age range, 24–53 years), who underwent preoperative 16-slice MDCT angiography and open donor nephrectomy, during a 31-month period from April 2005 to October 2007 at our institution. The hospital ethical committee approved our retrospective study, for which informed patient consent was not required.
MDCT scanning protocol
All MDCT studies were performed by using a 16-row MDCT scanner (Lightspeed Ultra, General Electrical Medical Systems, Milwaukee, WI, USA) with the same protocol consisting of arterial and nephrographic phases followed with conventional abdominal radiography. The subjects were instructed not to eat or drink anything for 3 h before the examination; no oral contrast material was administered. First, an initial scout topogram was obtained. Subsequently, 100–140 ml of nonionic iodinated contrast agent (Iodixanol, Visipaque 320 mgI/ml, GE Healthcare, Milwaukee, WI, USA) was injected through an 18–20 gauge cannula positioned in an antecubital vein at a flow rate of 4 ml/s using a power injector. The start time of arterial phase scanning was determined using automatic bolus tracking (Smart Prep, GE Healthcare). Scanning was initiated 5 s after a threshold of 125 HU was reached in the region of interest in the abdominal aorta. The area scanned extended from diaphragm to midsacrum. The arterial phase acquisition delays were 18–27 s, after the start of contrast injection. The main acquisition parameters for arterial phase were: the detector collimation of 16 × 0.625 mm, section thickness of 1.25 mm, intersection spacing of 0.625 mm, tube voltage of 120 kv, tube current of 200–240 mAs, table speed of 9.37 mm per rotation, gantry rotation time of 0.5 s. Nephrographic phase images were then acquired 85 s after the contrast injection using table speed of 18.75 mm per rotation, section thickness of 2.5 mm and intersection spacing of 1.25 mm. For the excretory phase, conventional radiography of the abdomen and pelvis was performed 5–10 min after contrast agent injection to define the collecting systems and ureters. All images were reconstructed with a standard soft tissue algorithm and transferred to a separate workstation for postprocessing.
Image analysis
For three-dimensional image reconstruction, the volumetric CT data sets were processed on a separate workstation (Advanced Workstation 4.2, GE Healthcare, Milwaukee, WI, USA) with multiplanar reformatting (MPR), curved planar reformatting (CPR), maximum intensity projection (MIP) and volume rendering (VR). Axial source images and the two and three-dimensional data sets for each of the 59 donors were evaluated by one of the three abdominal imaging radiologists who was blinded to surgical findings. All three radiologists documented the acceptability of technical quality of the MDCT studies in this report. For three-dimensional MDCT angiography, VR techniques were usually used, but MPR and MIP images were also used, especially for evaluation of small vessels. Renal arterial and venous anatomy was assessed primarily on arterial phase images, but if the renal veins, especially accessory draining renal veins including lumbar and gonadal veins, were not enhanced on the arterial phase images, nephrographic phase images were used.
The renal arterial anatomy was assessed for the number and branching patterns of arteries and the presence of arterial abnormalities. When the kidney had two or more arteries that had a separate aortic ostium, the vessel with the greatest diameter was considered to be main renal artery and others, accessory. An accessory artery was categorized according to its course as either polar (piercing the kidney directly) or hilar (entering the kidney at the hilum). Any branch diverged within 2.0 cm from the lateral wall the aorta in the left kidney or in retrocaval segment in the right kidney was classified as an early branching renal artery. For each renal artery, other associated findings, including the presence of stenosis, mural calcification, and beading pattern related to presumed fibromuscular dysplasia, were investigated. Renal venous anatomy was evaluated for the number of the renal veins and their anastomotic pattern. Venous anomalies were classified as major and minor. Major renal venous anomalies included the accessory renal veins, late venous confluence, circumaortic or retroaortic left renal veins and left sided or duplicated inferior vena cava. The late venous confluence was diagnosed on the left side when venous branches joined within 1.5 cm from the left lateral wall of abdominal aorta and on the right side when venous branches joined within 1.5 cm of the confluence with the inferior vena cava. Minor renal venous renal anomalies included the presence of draining gonadal or lumbar veins (especially those >5 mm in diameter) and drainage of any associated renal tributaries into these veins. Nephrographic phase images were primarily used to detect and characterize renal lesions. Excretory phase conventional radiography was used to assess the anatomy and associated abnormalities of the collecting systems and ureters.
Surgical correlation
All 59 subjects underwent donor nephrectomy using open extraperitoneal approach (55 left, 4 right). Surgery was performed between 4 weeks and 10 months after the MDCT angiography examination. The donated kidneys were selected on the basis of the MDCT angiography reports. The presence or absence of complex vascular anatomy was the principal consideration, and if the vascular anatomy was simple, the left kidney was preferred for donation due to the longer length of its veins. Donor nephrectomies were performed by one of the three transplant surgeons with 7–15 years of experience in renal transplantation surgery. The surgeons recorded a surgical result for each patient, including the number of arteries, the presence of early branching arteries, the number of renal veins, and the presence of major or minor renal vein anomalies.
Statistical analysis
Considering surgical findings as the reference standard, sensitivity, specificity, and accuracy of MDCT angiography to detect accessory renal arteries and veins, renal arteries with early branching and renal venous anomalies were calculated per donated kidney.
Results
The MDCT angiographic images were evaluated to be technically satisfactory in all 59 subjects. Fifty-nine of 118 kidneys were donated for transplantation; therefore, surgical confirmation of MDCT findings was obtained in these 59 kidneys. The renal arteries and veins were adequately enhanced on arterial phase images in all patients. The renal arteries could be easily differentiated from renal veins because arteries showed stronger enhancement than did veins. On the basis of MDCT angiography findings, 55 subjects (93%) underwent left nephrectomy and four subjects (7%) underwent right nephrectomy. Right nephrectomy was performed in these four subjects as the preoperative MDCT angiography showed three left renal arteries in three subjects and four left renal arteries in one subject.
Renal arteries
Sixteen of 59 (27%) kidneys showed surgically relevant anatomic variants of renal arteries. Among those, 9 (15%) kidneys with one accessory artery and 7 (12%) kidneys with early branching arteries were observed. During surgery, a total of 68 renal arteries were identified in 59 donor kidneys. In 50 (85%) donor kidneys one renal artery (Figs. 1, 2) and in 9 (15%) donor kidneys two arteries (Fig. 3) were identified. Three of nine accessory renal arteries were polar and six hilar. When compared with the surgical findings, 16-slice MDCT correctly identified all of the 68 renal arteries in 59 donor kidneys, with sensitivity and specificity of 100%. Early branching of the renal arteries was present in 7 (10%) of 68 renal arteries at surgery (Fig. 4). Sixteen-slice MDCT correctly diagnosed all of the seven early branching renal arteries with sensitivity and specificity of 100%. Arterial abnormalities were detected in four donors. Three of four cases had calcifications at the ostium of main renal arteries without significant luminal stenosis and one case of mild fibromuscular dysplasia of the right renal artery.
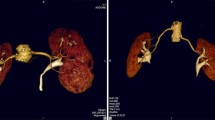
A 45-year-old woman who underwent left donor nephrectomy. Coronal volume rendering (a) and maximum intensity projection (b) images show one renal artery to each kidney. Axial (c) and sagittal multiplanar reformatted (d) images show a large lumbar vein (short arrow) draining into left renal vein (long arrow)
Renal veins
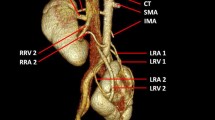
At surgery, 12 of 59 (20%) donor kidneys showed major renal venous anomalies including three accessory right renal veins (Fig. 5), four late venous confluence (2 right, 2 left) (Fig. 6), two circumaortic left renal veins (Figs. 7, 8) and three retroaortic left renal veins (Fig. 9). Sixteen-slice MDCT enabled a correct diagnosis in all cases of major renal venous anomalies with sensitivity and specificity of 100%.
A 49-year-old man who underwent left donor nephrectomy. Axial (a) and coronal multiplanar reformatted (b) images obtained during the nephrographic phase show retroaortic left renal vein (asterisk). Adrenal (short arrow) and gonadal vein (long arrow) tributaries to the left renal vein are seen in image (b). Ao aorta, IVC inferior vena cava
Twenty-nine minor left-sided venous anomalies were identified in 22 left kidneys at surgery including prominent (>5 mm) gonadal veins draining into left renal vein (Figs. 10, 11, 12) in 15 cases, prominent (>5 mm) lumbar veins draining into left renal vein (Figs. 1, 10, 12) in 12 cases, small renal venous branch draining into gonadal vein in one cases (Fig. 12) and small renal venous branch draining into the lumbar vein in one cases. Sixteen-slice MDCT correctly diagnosed 23 of 29 (sensitivity 79%) cases of minor renal venous anomalies. Two cases of small renal venous branch one of which drained into the gonadal vein (Fig. 12) and one of which into lumbar vein, and four cases of a prominent lumbar vein draining into the left renal vein were missed by MDCT.
A 41-year-old man who underwent left donor nephrectomy. Coronal volume rendering (a), maximum intensity projection (b), and curved multiplanar reformatted (c) images show an accessory renal artery (arrow) to the lower pole of the right kidney and early branching of the main right renal artery. A lumbar vein (LV) and gonadal vein (GV) draining into left renal vein (LRV) are also seen
A 51-year-old man who underwent left donor nephrectomy. Coronal volume rendering (a) and maximum intensity projection (b, c) images reveal one accessory renal artery (arrow) and two accessory renal veins (asterisks) on the right. Curved multiplanar reformatted image (d) shows gonadal vein (GV) connecting with left renal vein (LRV). RRV right main renal vein, IVC inferior vena cava
A 35-year-old woman who underwent left donor nephrectomy. Coronal volume rendering image shows an accessory right renal artery (long arrow) which entering hilum of the kidney and a left gonadal vein (GV) draining into left renal vein (LRV). A lumbar vein (LV) and small venous branch (short arrow) which connect to left gonadal vein are also present. The small venous branch was missed by MDCT due to its small diameter
Renal parenchyma
Renal parenchyma of both kidneys were adequately enhanced on nephrographic phase images in all patients. Nine kidneys had simple renal cysts that were smaller than 1 cm in diameter, two kidneys had parenchymal scars, and one kidney had a renal calculus.
Pelvicalyceal system and ureters
At surgery, 56 of 59 kidneys had a normal single ureter, two of partial ureteral duplication in the right kidneys and one of complete ureteral duplication in the left kidney. At excretory phase conventional abdominal radiography, the pelvicalyceal systems and ureters were adequately opacified in all patients. All of three ureteral duplications were correctly identified at excretory phase by conventional abdominal radiography (sensitivity 100%).
Discussion
Potential living renal donors should undergo comprehensive preoperative assessment including clinical evaluation, laboratory test, and diagnostic imaging. By tradition, potential living renal donors are assessed with a combination of excretory urography and renal catheter angiography. Angiography was performed to identify the number of renal arteries and presence of prehilar branching and any vascular disease. But, it has limited value in detailed evaluation of complex renal venous anomalies. Moreover, it is expensive, has serious complications such as intima injury, dissection and subsequent stenosis of the vessel [2].
Several studies have demonstrated that spiral CT angiography can replace excretory urography and renal catheter angiography [12, 17, 20]. Preoperative CT evaluation of potential renal donors may depict the presence of renal arterial and venous variants, abnormalities of the renal parenchyma and collecting system, renal calculi, and other renal and extrarenal abnormalities. Three-dimensional spiral CT angiography has been reported to be as accurate as renal angiography for evaluation of arterial anatomy and more sensitive than renal angiography and intravenous urography in assessment of the venous anatomy and parenchyma. The reported accuracy of spiral CT angiography in detecting accessory arteries, prehilar branching, and renal venous anatomy is in the range of 78–98, 89–99, and 90–99%, respectively [12, 17, 20].
The rapid evolution of CT technology and the introduction of MDCT have provided shorter image acquisition times, reduced tube heating, narrower collimation, and improved spatial resolution, compared with single-detector spiral CT [19]. MDCT scanners are particularly useful for angiographic applications because they provide larger anatomic coverage, increased contrast enhancement of the arteries, and higher longitudinal spatial resolution, as well as more detailed and sensitive depiction of the renal vasculature. The larger volume of coverage is important for preoperative evaluation of living kidney donors due to the fact that accessory renal arteries may arise from the distal abdominal aorta or common iliac arteries. Currently, MDCT angiography has become a noninvasive alternative imaging modality to catheter renal angiography for evaluation of renal vascular anatomy and variants [3, 5, 6, 8, 14–16, 18].
Magnetic resonance (MR) angiography is an acceptable alternative noninvasive imaging modality in donors since it has the added benefits of no iodinated contrast material and no exposure to ionizing radiation [4, 9]. However, MR imaging is not sensitive in detection of urolithiasis, and its spatial resolution is inferior to that of MDCT. Less common availability and higher cost are other limitations of MR imaging. Gadolinium-enhanced MR angiography for evaluation of accessory arteries has been shown to have a sensitivity, specificity, and accuracy of 89, 94, and 91%, respectively [4].
By and large, the kidneys are supplied by single renal arteries that arise from the abdominal aorta at the level of upper margin of the second lumbar vertebral body. In about 32% of cases, there are multiple renal arteries unilaterally and in 15% of cases bilaterally. The most common accessory artery arises from the abdominal aorta and supplies the inferior renal pole [13]. The most common renal venous anomaly is the occurence of multiple renal veins, which can be seen in up to 15–30% of patients, commonly on the right side. Circumaortic and retroaortic left renal veins occur in up to 17 and 3% of patients, respectively [1, 13]. Conditions precluding donation include unilateral agenesis, congenital fusion anomalies, bilateral multiple (>3) arteries or veins, bilateral arterial or venous aberrant supply or drainage (e.g., iliac vessels), diffuse renal disease, renal neoplasm, hydronephrosis, medullary sponge kidney and renal papillary necrosis [18]. Usually, the left kidney is harvested because of the longer length of the renal vein; however, in case of multiple renal vessels on the left, the right kidney is preferred. Therefore, determination of the detailed anatomy of the accessory renal vessels is an important factor in the selection of donor kidney.
Ionizing radiation exposure of healthy kidney donors should be considered when using MDCT angiography, although, the total radiation dose for a triple-phase MDCT was shown to be less than that with the combination of renal angiography and excretory urography [18]. To minimize the dose of ionizing radiation, we performed dual-phase MDCT including arterial phase and nephrographic phase, obtained with 85 s delay. In addition, excretory phase conventional abdominal radiography was used to assess the collecting systems and ureters. We did not perform precontrast, venous or excretory phase MDCT. Unenhanced CT is used to localize the kidneys, for the detection of urolithiasis, and obtain a baseline attenuation measurement for the evaluation of incidental renal masses. But the presence of urolithiasis can be easily detected on arterial phase images. Although most of authors prefer the venous phase, acquired usually 40–55 s after the contrast agent injection, we did not perform venous phase because major variants in the renal venous system are well depicted during the arterial phase, but systemic tributaries to the renal veins are not well opacified during the venous phase; therefore, we preferred nephrographic phase. We preferred a conventional abdominal radiography rather than delayed phase MDCT because it provides sufficient information on pelvicalyceal systems and ureters at a lower radiation dose.
Preoperative CT angiography of potential renal donors enables definition of the renal venous anatomy. The reported accuracy of MDCT in the evaluation of renal venous anatomy ranges from 93 to 100% in evaluation of major renal venous anomalies [6, 8]. Although some surgeons consider the presence of accessory veins of minor importance for their surgical strategy and management, a reliable visualization and knowledge of renal venous anatomy may still be considered helpful especially in laparoscopic nephrectomy. Identification of minor renal venous renal anomalies including the presence of draining prominent (>5 mm) gonadal or lumbar veins and drainage of any associated small renal vein branch into these veins have not been well characterized by imaging [14]. The relationships among renal, gonadal, and lumbar veins are variable. Accessory renal vein branches usually drain to gonadal or lumbar veins before joining the main renal vein. One or more lumbar veins join with the left renal vein in approximately 43–75% of patients [10]. The preoperative diagnosis of these minor renal venous anomalies are important, especially in laparoscopic donor nephrectomy as the posterior aspect of the renal vein often can not be directly visualized during surgery. MDCT protocols should be optimized to maximize enhancement of these small vessels because identifying some variants can be difficult because of their complexity and variability [14, 15]. We evaluated renal venous anatomy primarily on arterial phase images because renal veins quickly enhance and are usually visible on arterial phase images, but if the renal veins were not enhanced on the arterial phase images, nephrographic phase images were used. Our sensitivity for identification of minor renal venous anomalies was 79% (23 of 29). Two cases of small renal venous branch, one of which drained into the gonadal vein and one of which into lumbar vein, and four cases of a prominent lumbar vein draining into the left renal vein were missed by MDCT probably due to their small diameters and insufficient opacification of these vessels in both arterial and nephrographic phases.
In this study, we had an excellent sensitivity for the detection of accessory arteries and early arterial branching, with sensitivity and accuracy of 100%. Our sensitivity and accuracy for detection of major renal venous anomalies including the accessory renal veins, late venous confluence, circumaortic or retroaortic left renal veins were 100%. However, the sensitivity for the identification of minor renal venous anomalies including draining gonadal or lumbar veins and drainage of any associated small renal venous branch into these veins was 79%.
There were some limitations in our study. First, the surgeons preferentially removed the kidney that had less complicated vascular anatomy. Therefore, the kidneys with more complex anatomy had no pathologic proof, and the accuracy rates were given only for the less complicated kidneys. Second, this was a retrospective study, in which all the MDCT studies were reviewed by only one of the three radiologists and not independently by all of the three radiologists.
Conclusion
In conclusion, 16-slice MDCT is a fast and noninvasive imaging modality that provides highly accurate and detailed evaluation of renal arterial and venous anatomy in potential living renal donors. The dose of ionizing radiation of healthy kidney donors should be kept minimum. Dual-phase MDCT angiography including arterial and nephrographic phases followed by conventional abdominal radiography enables complete assessment of the potential living renal donors without significant increase of radiation dose. However, the evaluation of minor venous variants may be problematic because of their small diameters and poor opacification in both arterial and nephrographic phases.
References
Abrams HL, Faitelson BB (1983) Renal venography. In: Abrams HL (ed) Abrams angiography. Little Brown, Boston, pp 1290–1324
Hänninen EL, Denecke T, Stelter L et al (2005) Preoperative evaluation of living kidney donors using multirow detector computed tomography: comparison with digital substraction angiography and intraoperative findings. Transpl Int 18:1134–1141
Holden A, Smith A, Dukes P, Pilmore H, Yasutomi M (2005) Assessment of 100 live potential renal donors for laparoscopic nephrectomy with multi-detector row helical CT. Radiology 237:973–980
Jha RC, Korangy SJ, Ascher SM, Takahama J, Kuo PC, Johnson LB (2002) MR angiography and preoperative evaluation for laparoscopic donor nephrectomy. AJR Am J Roentgenol 178:1489–1495
Kapoor A, Mahajan G, Singh A, Sarin P (2004) Multispiral computed tomographic angiography of renal arteries of live potential renal donors: a review of 118 cases. Transplantation 77:1535–1539
Kawamoto S, Montgomery RA, Lawler LP, Horton KM, Fishman EK (2003) Multidetector CT angiography for preoperative evaluation of living laparoscopic kidney donors. AJR Am J Roentgenol 180:1633–1638
Kawamoto S, Lawler LP, Fishman EK (2005) Evaluation of the renal venous system on late arterial and venous phase images with MDCT angiography in potential living laparoscopic renal donors. AJR Am J Roentgenol 184:539–545
Kim JK, Park SY, Kim HJ et al (2003) Living donor kidneys: usefulness of multi-detector row CT for comprehensive evaluation. Radiology 229:869–876
Kim T, Murakami T, Takahashi S et al (2006) Evaluation of renal arteries in living renal donors: comparison between MDCT angiography and gadolinium-enhanced 3D MR angiography. Radiat Med 24:617–624
Lin CH, Steinberg AP, Ramani AP et al (2004) Laparoscopic live donor nephrectomy in the presence of circumaortic or retroaortic left renal vein. J Urol 171:44–46
Nicholson ML, Bradley JA (1999) Renal transplantation from living donors: should be seriously considered to help overcome the shortfall in organs. Br Med J 318:409–410
Platt JF, Ellis JH, Korobkin M, Reige K (1997) Helical CT evaluation of potential kidney donors: findings in 154 subjects. AJR Am J Roentgenol 169:1325–1330
Pollak R, Prusak BF, Mozes MF (1986) Anatomic abnormalities of cadaver kidneys produced for purposes of transplantation. Am Surg 52:233–235
Raman SS, Pojchamarnwiputh S, Muangsomboon K, Schulam PG, Gritsch HA, Lu DS (2006) Utility of 16-MDCT angiography for comprehensive preoperative vascular evaluation of laparoscopic renal donors. AJR Am J Roentgenol 186:1630–1638
Raman SS, Pojchamarnwiputh S, Muangsomboon K, Schulam PG, Gritsch HA, Lu DS (2007) Surgically relevant normal and variant renal parenchymal and vascular anatomy in preoperative 16-MDCT evaluation of potential laparoscopic renal donors. AJR Am J Roentgenol 188:105–114
Rastogi N, Sahani DV, Blake MA, Ko DC, Mueller PR (2006) Evaluation of living renal donors: accuracy of three-dimensional 16-section CT. Radiology 240:136–144
Rubin GD, Alfrey EJ, Dake MD et al (1995) Assessment of living renal donors with spiral CT. Radiology 195:457–462
Rydberg J, Kopecky KK, Tann M et al (2001) Evaluation of prospective living renal donors for laparoscopic nephrectomy with multisection CT: the marriage of minimally invasive imaging with minimally invasive surgery. Radiographics 21:S223–S236
Rydberg J, Liang Y, Teague SD (2003) Fundamentals of multichannel CT. Radiol Clin North Am 41:465–474
Smith PA, Ratner LE, Lynch FC, Corl FM, Fishman EK (1998) Role of CT angiography in the preoperative evaluation for laparoscopic nephrectomy. Radiographics 18:589–601
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Türkvatan, A., Akıncı, S., Yıldız, Ş. et al. Multidetector computed tomography for preoperative evaluation of vascular anatomy in living renal donors. Surg Radiol Anat 31, 227–235 (2009). https://doi.org/10.1007/s00276-008-0428-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-008-0428-0