Abstract
In order to elucidate the lymphatic pathways from the thoracic esophagus, minute dissection of five adult cadavers, from the neck through the diaphragm, was performed. Peri-esophageal lymphatics were dissected from both the anterior and posterior aspects. The topographical differences between the right and left lymphatic drainage were revealed. The right lymphatic drainage system (RDS) was basically longitudinal and multi-stationed. Longitudinal lymphatics were relatively poorly developed in the left lymphatic drainage system (LDS), and direct drainage to the thoracic duct from the left wall of the thoracic esophagus, was frequently observed. The right uppermost thoracic paratracheal node received almost all levels of the right esophageal wall, and this node was thought to be the key node in the RDS. A contralateral lymphatic pathway was relatively frequently observed in the middle and lower thoracic esophagus. These results seemed to be in agreement with the anatomical and clinicopathological data in the literature, and might serve as a basis for sentinel node navigation surgery for the thoracic esophageal cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis of thoracic esophageal cancer has improved since the prevalence of the three-field dissection in esophageal cancer surgery [1, 5, 29]. Lymph node metastasis has been known to be a prime prognostic factor [17, 27]. Recently, sentinel node navigation surgery for thoracic esophageal cancer has been adopted in influential institutions with rather preferable outcomes [8, 13]. It is crucial to know the precise anatomical location and connections of the widespread and complicated lymph nodes and lymphatic vessels associated with the drainage from the thoracic esophagus, to achieve an adequate field of dissection for each case. In this study, the cervical through thoracic lymphatics concerned with the drainage from the thoracic esophagus were studied by minute cadaveric dissections. Here, we discuss the lymphatic flow from the thoracic esophagus with analysis of the anatomical and clinicopathologic data in the literature.
Materials and methods
Five adult embalmed cadavers (obtained for routine dissection), free from head and neck or thoracic or abdominal malignant tumors (Table 1), were used. After the bony structure of the thorax was removed, the cervical visceral organs through the thoracic organs with mediastinum, including the hypopharynx, larynx, cervical to thoracic esophagus, thyroid gland, trachea, heart, bilateral lungs and diaphragm were removed en block. In order to distinguish the lymph vessels, the arteries and veins were initially traced under magnification using a binocular loupe, following which the lymph vessels were identified [23]. Every specimen was first approached anteriorly, identifying the lymph nodes and lymphatic vessels around the trachea, the bilateral bronchi, aortic arch, and superior vena cava with it’s jugular and subclavicular branches. Then the specimens were approached posteriorly, with the lymph nodes and lymphatic vessels around the esophagus and posterior mediastinum being identified. Consequently, the lymph nodes and lymphatic vessels all around the cervical through thoracic esophagus were completely dissected, and their relationships to the lymphatics around the airway and major blood vessels were clarified. Then specimens were observed posteriorly, and were sketched from two different aspects, the right posterior oblique and the left posterior oblique view, in order to precisely describe the bilateral esophageal lymphatics. In specimen 3, however, bilateral lymphatics were observed almost completely in the right posterior oblique view, and were described only from that aspect. In order to elucidate the lymphatic pathways, the esophagus was pulled to the left with the trachea and bronchi shifted to the right in the right posterior oblique view; the esophagus was pulled to the right with the trachea and bronchi shifted to the left in the left posterior oblique view. The drawings were scanned and processed on a personal computer, and a detailed image was obtained again using the Adobe Photoshop version 7.0. Finally, the lymphatic connection toward the bilateral venous angle from the cervicothoracic esophagus and anatomical sentinel nodes was studied. A considerable time-consuming effort was necessary for the minute dissections of the wide lymphatic area from the neck through the diaphragm. Although cervico-thoracic-abdominal dissection is properly required for the complete description of the efferent lymphatics from the thoracic esophagus, abdominal dissection was not performed in this study due to the difficulties of additional dissections of the abdomen, in which the lymphatics were related to many organs. Accordingly, abdominal lymphatics from the thoracic esophagus will be studied further in the future.
Definitions
The border of the cervical and thoracic esophagus is defined at the level of the jugulo-subclavian confluence (JSC), because the bony structure of the thorax was removed. The border of the hypopharyx and the cervical esophagus is defined at the level of the inferior border of the cricoid cartilage. The thoracic esophagus includes that from JSC to the diaphragm and is subdivided as follows (following in part, the classification by the Japanese Society for Esophageal Diseases [7]). If the midpoint between the JSC and the carina, and the midpoint between the carina and the diaphragm are termed M and N, respectively, the thoracic uppermost esophagus includes that from JSC to M; thoracic upper esophagus, from M to the carina; thoracic middle esophagus, from the carina to N; thoracic lower esophagus, from N to the diaphragm. This classification was employed based on the fact that the lymphatic drainage from the uppermost and the upper thoracic esophagus is somewhat different as described below.
In this paper, the efferent lymphatics from the cervicothoracic esophagus toward the bilateral venous angle (VA) have been studied. The lymph nodes around the airway are situated ventral to the periesophageal nodes and are closer to the VA. Therefore, if the efferent lymphatic vessels or periesophageal nodes were connected to the nodes around the airway, the lymphatics were postulated to flow in a direction from the esophagus to the airway. It has been reported that the efferent lymphatics from the thoracic esophagus flow both superiorly and inferiorly, however, in this study, we only examined the ascending flow because the intraabdominal lymphatics were not dissected. Consequently, the longitudinal lymphatics on the adventitia of the esophagus and those around the trachea were postulated to ascend. The organs concerned with the drainage from the thoracic esophagus are the lymph nodes, lymphatic vessels and the thoracic duct. If a lymph node received the first efferent drainage vessel from an organ, it was termed an anatomical sentinel node. When a lymph node was situated between the esophagus and the membranous portion of the trachea, it was termed as a paraesophageal node or a retrotracheal node due to its closer position to the esophagus or the trachea.
Results
Intrathoracic lymphatic structure associated with drainage from the thoracic esophagus
In the mediastinum, several organs such as the thoracic esophagus, the trachea, the bilateral bronchi, the thoracic aorta and the heart were located in proximity to each other. The efferent lymphatics from the thoracic esophagus were associated with the lymphatics from these organs.
Paraesophageal nodes (E)
Paraesophageal nodes were thought to be situated in the vicinity of the esophagus, however, they were not evenly distributed on each level of the esophagus, and tended more frequently to be located on the middle and lower thoracic esophagus (Figs. 1, 2, 3, 4, 5).
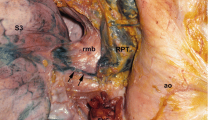
Illustration of specimen 1. a Right posterior oblique view. Thoracic para-aortic lymphatic chain (AC) was situated between the esophagus and the descending aorta. b Left posterior oblique view (see text). A Thoracic paraaortic node, AC thoracic paraaortic lymph chain, Az azygos vein, B bifurcational node, BA broncheal artery, BCV brachiocephalic vein, C cervical paratracheal node, D-Ao descending aorta, E paraesophageal node, EJV external jugular vein, Eso esophagus, IJV internal jugular vein, J deep cervical node, l left, M main bronchus node, P thoracic paratracheal node, PM posterior mediastinal node, R retrotracheal node, r right, RLN recurrent laryngeal node, SCV subclavian vein, TD thoracic duct, U uppermost thoracic paratracheal node, VA venous angle
Bifurcational nodes (B) and main bronchus nodes (M)
The bifurcational nodes (B) were those situated at the bifurcation of the trachea, and the main bronchus nodes (M) were those situated in the proximity of the hilus of the lungs. These nodes were concerned with the drainage of the lungs, however, it was recognized in every specimen, that the efferent lymphatics from the middle or lower thoracic esophagus connected to B or M, directly or via the paraesophageal nodes (Figs. 1, 2, 3, 4, 5).
Tracheobroncheal nodes (T)
These nodes were located on the lateral wall of the tracheal bifurcation. The right tracheobroncheal node was situated in the vicinity of the confluence of the azygos vein to the superior vena cava. It was considered to relay not only the lymphatics from the airway, but also those from the upper thoracic esophagus (Figs. 1, 2, 3, 4, 5).
Retrotracheal nodes (R)
The retrotracheal nodes were defined as those behind the trachea. It was considered to drain both the trachea and the esophagus. It seemed to drain the esophagus in two ways: draining the middle and the lower thoracic esophagus by relaying to the lymphatics from the bifurcational node and those toward the right uppermost paratracheal node (Figs. 2, 3), and by draining the upper thoracic esophagus intercalating between the esophageal wall and the tracheobroncheal node (Figs. 2, 3). In the latter, R tended to be situated in the vicinity of the right broncheal artery (Figs. 2, 3).
Illustration of specimen 2. a Right posterior oblique view. Right wall of the esophagus was pulled to the left with thread. A well-developed lymphatic plexus was observed in the right cervicothoracic region in this specimen. b Left posterior oblique view. Lymphatics from the right wall of the lower thoracic esophagus ran contralaterally and joined the thoracic duct via a posterior mediastinal node (PM3). A Thoracic paraaortic node, AC thoracic paraaortic lymph chain, Az azygos vein, B bifurcational node, BA broncheal artery, BCV brachiocephalic vein, C cervical paratracheal node, D-Ao descending aorta, E paraesophageal node, EJV external jugular vein, Eso esophagus, IJV internal jugular vein, J deep cervical node, l left, M main bronchus node, P thoracic paratracheal node, PM posterior mediastinal node, R retrotracheal node, r right, RLN recurrent laryngeal node, SCV subclavian vein, TD thoracic duct, U uppermost thoracic paratracheal node, VA venous angle
Illustration of specimen 3 (see text). A Thoracic paraaortic node, AC thoracic paraaortic lymph chain, Az azygos vein, B bifurcational node, BA broncheal artery, BCV brachiocephalic vein, C cervical paratracheal node, D-Ao descending aorta, E paraesophageal node, EJV external jugular vein, Eso esophagus, IJV internal jugular vein, J deep cervical node, l left, M main bronchus node, P thoracic paratracheal node, PM posterior mediastinal node, R retrotracheal node, r right, RLN recurrent laryngeal node, SCV subclavian vein, TD thoracic duct, U uppermost thoracic paratracheal node, VA venous angle
Paratracheal nodes (C and P), and right uppermost paratracheal nodes (U)
The paratracheal nodes were those located on both sides of the trachea, and were subdivided to cervical (C) or thoracic (P) by their level of existence. Some of them were situated along the recurrent laryngeal nerves (RLN) and were considered to belong to the recurrent nerve chain (RN) [31].
All the left P were found along the left RLN, and were distributed almost evenly (Figs. 1, 2, 4). The right P were distributed in two ways: the right lower P existed in front of the vagus nerve, while the right upper P was found behind or lateral to the right RLN, somewhat apart from the right lower P (Figs. 1, 2, 3, 4, 5). The right upper P was termed the right uppermost paratracheal node (U), while the right lower P was called the right P here. Hence the right RN coincided with the right U, while the left RN, with the left P.
Illustration of specimen 4. a Right posterior oblique view. Right wall of the esophagus was pulled to the left and the right subclavian artery was pulled down with thread. b Left posterior oblique view. A left paratracheal lymphatic pathway was well developed in this specimen. A Thoracic paraaortic node, AC thoracic paraaortic lymph chain, Az azygos vein, B bifurcational node, BA broncheal artery, BCV brachiocephalic vein, C cervical paratracheal node, D-Ao descending aorta, E paraesophageal node, EJV external jugular vein, Eso esophagus, IJV internal jugular vein, J deep cervical node, l left, M main bronchus node, P thoracic paratracheal node, PM posterior mediastinal node, R retrotracheal node, r right, RLN recurrent laryngeal node, SCV subclavian vein, TD thoracic duct, U uppermost thoracic paratracheal node, VA venous angle
Illustration of specimen 5. a Right posterior oblique view. Longitudinally running extramural collecting lymphatic vessels along the esophagus and pericardium were noted. b Left posterior oblique view. Collecting lymphatic vessels from the right and left esophageal wall directly drained into the thoracic duct (TD). A Thoracic paraaortic node, AC thoracic paraaortic lymph chain, Az azygos vein, B bifurcational node, BA broncheal artery, BCV brachiocephalic vein, C cervical paratracheal node, D-Ao descending aorta, E paraesophageal node, EJV external jugular vein, Eso esophagus, IJV internal jugular vein, J deep cervical node, l left, M main bronchus node, P thoracic paratracheal node, PM posterior mediastinal node, R retrotracheal node, r right, RLN recurrent laryngeal node, SCV subclavian vein, TD thoracic duct, U uppermost thoracic paratracheal node, VA venous angle
Thoracic paraaortic nodes (A) and thoracic paraaortic lymph chain
The thoracic duct existed between the esophagus and the descending aorta. A lymphatic pathway on the descending aorta was observed in four out of five specimens (Figs. 1, 2, 3, 4). The nodes and the lymphatic pathway on the thoracic aorta were termed the thoracic paraaortic nodes (A) and thoracic paraaortic lymph chain (AC). In specimen 1, the AC was located between the thoracic duct and the esophagus (Fig. 1), while in specimens 2–4, it was lateral to the thoracic duct (Figs. 2, 3, 4). In specimens 1, 2 and 4, several A accompanied AC, while in specimen 3, only the lymphatic vessels were observed (Figs. 1, 2, 3, 4). The AC terminated in the thoracic duct, and received lymphatics from the esophagus in specimens 1–3, while in specimen 4, AC seemed to be independent of the esophagus (Figs. 1, 2, 3, 4).
Posterior mediastinal nodes (PM)
PM have been defined as the nodes along the descending aorta, inferior pulmonary veins and the pericardium by the Japanese Society for Esophageal Diseases [7]. In this study, the nodes on the pericardium, or those adjacent to the thoracic duct were included (Figs. 2, 4, 5).
Deep cervical nodes or internal jugular chain nodes (J) and the jugular trunk
Deep cervical nodes or internal jugular chain nodes were those along the internal jugular vein and the carotid arteries. The jugular trunk is the vessel, which collects lymph from J, and on the right, it terminates in the jugulosubclavian junction or the right lymphatic duct; on the left, it usually terminates in the thoracic duct but it may join the internal jugular vein or subclavian vein [2]. In specimen 3, the lymphatics on the right to posterior wall of the uppermost thoracic esophagus drained directly to J (Fig. 3).
Extramural collecting lymphatic vessels
Drainage from the middle and lower thoracic esophagus
(1) Specimen 1 (Fig. 1a, b): Bifurcational node (B1) received lymphatics from the right wall of the middle and lower thoracic esophagus and received vessels from the right main bronchus node (M1), which drained the right lung. The ascending vessels from B2, another bifurcational node continuous with B1, ran mainly longitudinally joining the vessels from the right wall of the upper and uppermost thoracic esophagus, and partly ran somewhat antero-laterally to reach the tracheobroncheal nodes (T1–2), which were continuous with the right thoracic paratracheal nodes (P1–3). The left lower paraesophageal node (E) drained the left wall of the lower thoracic esophagus, and joined with the thoracic paraaortic lymph chain (AC). AC also directly drained the left wall of the middle thoracic esophagus and terminated in the thoracic duct.
(2) Specimen 2 (Fig 2a, b): The right lower paraesophageal nodes (E1–2) drained the right wall of the lower and middle thoracic esophagus and joined the vessels from the diaphragm. Most of the efferents from E2 ran contralaterally to the posterior mediastinal node (PM3), which was intercalated on the way to the thoracic duct, and some efferents ran to a bifurcational node (B1). The left wall of the lower thoracic esophagus was mainly drained by a thoracic paraaortic node (A3), the efferent of which joined the thoracic duct via the thoracic paraaortic lymph chain (AC). The left wall of the middle thoracic esophagus was drained by a series of paraesophageal nodes (E3–5); the efferent of E5 reached a bifurcational node (B2). Efferent from B1 joined the efferent from B2 and terminated in the right main bronchus node (M1), the efferent of which ran to a right tracheobroncheal node (T). Upward efferent from B2 ran longitudinally intercalated by a series of retrotracheal nodes (R1–3), and finally to thoracic paratracheal nodes (P1–3) and the uppermost paratracheal node (U) through the lymphatic networks around the trachea at the thoracic inlet.
(3) Specimen 3 (Fig 3a, b): The right wall of the lower, and the middle thoracic esophagus was drained by a lower paraesophageal node (E1), and a bifurcational node (B1), respectively, collecting vessels from the diaphragm. E1 connected to E2, a large middle thoracic paraesophageal node situated on the left wall of the middle thoracic esophagus. E2 connected to a thoracic paraaortic-collecting vessel (AC), on the way to the thoracic duct. Efferent from B1 was partly relayed longitudinally by retrotracheal nodes (R1–5), and partly traversed to a right tracheobroncheal node (T). A drainage vessel from the left wall of the middle thoracic esophagus joined AC.
(4) Specimen 4 (Fig. 4a, b): The right main bronchus nodes (M1–2) drained the right middle thoracic esophageal wall and were continuous with M3, another main bronchus node, the efferents of which connected with efferents from the right upper thoracic paraesophageal nodes (E2–3), and terminated in the right tracheobronchial nodes (T1–2). T1–2 connected with the right thoracic paratracheal nodes (P1–3). A left lower paraesophageal node (E1) drained the diaphragm and the left lower thoracic esophageal wall. Efferents from E1 ascended, relayed by a series of posterior mediastinal nodes (PM1–8) on the pericardium and terminated in the left main bronchus nodes (M4–6), the efferents from which converged to M7. Efferents from M7 ran, first to a left tracheobroncheal node (T3), then to left thoracic paratracheal nodes (P4–8). M6–7 drained the left wall of the middle thoracic esophageal wall. Efferents from the left posterior wall of the middle thoracic esophagus ran directly to the thoracic duct (Fig. 4b, white arrowhead). In this specimen, a well-developed thoracic paraaortic lymph chain (AC) was observed with several paraaortic lymph nodes (A1–4), which seemed to be independent of the thoracic esophagus.
(5) Specimen 5 (Fig. 5a, b): The right lower and middle thoracic paraesophageal nodes (E1–2) drained the esophageal wall of each level and connected with each other. A posterior mediastinal node (PM1) also drained the right wall of the lower thoracic esophageal wall, and efferents from E1 and PM1 formed a network, and terminated in bifurcational nodes (B1–2). The left wall of the lower thoracic esophagus was drained by a small paraesophageal node (E4), efferents from which ran to a left main bronchus node (M2), which was connected to B1, which was continuous to B2. Some efferents from B2 ran to a tracheobroncheal node (T), and then to a thoracic paratracheal node (P1). Some efferents from E2 connected to the thoracic duct, and some ascended longitudinally alongside the esophagus joining the efferents from B2 and terminated in a right uppermost thoracic paratracheal node (U) via a right upper thoracic paraesophageal node (E3).
Drainage from the upper and uppermost thoracic esophagus and terminal lymphatic flow toward the bilateral venous angle
(1) Specimen 1 (Fig. 1a, b): The right wall of the upper and uppermost thoracic esophagus was drained by a right uppermost thoracic paratracheal node (U), the efferents from which ran to the right venous angle (VA) joining efferents from B2. Most efferents from a right thoracic paratracheal node (P2) ascended to U; some efferents went directly to the right VA. The left wall of the upper and uppermost thoracic esophagus was drained by a tracheobroncheal node (T3) and a thoracic paratracheal node (P4). Some efferents from T3 and P4 connected to the thoracic duct (TD), and some ascended to P5, which was continuous with a cervical paratracheal node (C1). Efferents from C1 connected to the TD, which terminated in the left VA.
(2) Specimen 2 (Fig. 2a, b): The right wall of the upper thoracic esophagus was drained by retrotracheal nodes (R1–2) and a paraesophageal node (E6), efferents from these nodes ran to a right tracheobroncheal node (T). Some efferents from R2 ascended on the membranous portion of the trachea, joining efferents from R1, to which efferents from B2 connected. Well-developed lymphatic network draining the right wall of the uppermost thoracic and cervical esophagus was observed in this specimen. This network was associated with paratracheal nodes, which were distributed from the thoracic to cervical level (P1–5, U and C). P1–5 and U connected with each other, and U terminated in the right venous angle (VA). Efferents from C were associated with the deep cervical nodes (J1–3), and terminated in the right VA. The lymphatic vessels draining the left wall of the upper and uppermost thoracic esophagus were connected to the thoracic duct, which terminated in the left VA.
(3) Specimen 3 (Fig. 3a): The right wall of the upper thoracic esophagus was drained by a retrotracheal node (R3), the efferents of which partly traversed to a right tracheobroncheal node (T) and partly connected to R4, and the efferents from R4 ascended to R5 or to a right uppermost thoracic paratracheal node (U). The right wall of the uppermost thoracic esophagus was drained by either R5, or deep cervical nodes (J2, 4) rather distant from the original region. A series of retrotracheal nodes (R1–5) formed an ascending lymphatic connection behind the trachea. T was connected to a thoracic paratracheal node (P), the efferents of which joined with a vessel from R4 on the way to U. R5 was connected to U and J1. U was connected to J1, and the efferents from J1–4 were associated with a deep cervical node (J5) just above the right venous angle (VA) and terminated in the right VA. The drainage vessels from the left upper and uppermost thoracic esophagus were directly connected to the thoracic duct, which ran to the left VA.
(4) Specimen 4 (Fig. 4a, b): The right wall of the upper thoracic esophagus was drained by paraesophageal nodes (E2–3), the efferents from which joined a vessel from a main bronchus node (M3), on the way to tracheobroncheal nodes (T1–2). Right uppermost thoracic paratracheal nodes (U1–2) drained the right wall of the uppermost thoracic esophagus as well as the right wall of the trachea. Efferents from U2 ran to C1, a cervical paratracheal node. T1–2 were connected to thoracic paratracheal nodes (P1–3). In this specimen, P2–3 and U1–2 were continuous. Efferents from P2–3 were associated with deep cervical nodes (J1–3). A rather thick vessel from P3 connected to a right common jugular trunk just above the right venous angle (Fig. 4a, black arrowhead). J3 seemed to be a last echelon lymph node before termination of the deep cervical nodes to the common jugular trunk, and was associated with other deep cervical nodes, and received vessels from C1 and direct drainage vessels from the right wall of the cervical esophagus. Lymphatic vessels draining the posterior wall of the uppermost thoracic esophagus were directly connected to the thoracic duct (Fig. 4b, black arrowhead). The left wall of the uppermost thoracic esophagus was mainly drained by left thoracic paratracheal nodes (P4–6). P6 was continuous with P7, the efferent vessels from which connected to the thoracic duct just before its opening into the subclavian vein. A lymphatic vessel from the left wall of the cervical esophagus joined a vessel from the posterior wall of the meso-to-hypopharynx and connected to the left common jugular trunk before its opening into the jugulosubclavian confluence (Fig. 4b, asterisk).
(5) Specimen 5 (Fig. 5a, b): Longitudinal vessels continuous from the lower thoracic esophagus were associated with the drainage from the right upper and uppermost thoracic esophagus. These vessels terminated in the right uppermost thoracic paratracheal node (U) via a paraesophageal node (E3), as mentioned above. Several vessels draining the right wall of the uppermost thoracic and cervical esophagus were connected to U and the cervical paratracheal nodes (C1–3). Efferent vessels from the right thoracic paratracheal nodes (P1–4) ran to U. Efferent vessels from U and C1–2 joined the right jugular trunk just before its opening into the right venous angle (VA). The draining vessel from the left wall of the upper thoracic esophagus directly opened into the thoracic duct. The left wall of the cervical esophagus was drained by a left lowermost deep cervical node (J1), the efferents from which ran to the left VA.
Schematic representations of each specimen (Fig. 6)
The lymphatic pathways from the right and left wall of the esophagus seemed to be different morphologically, presumably due to the differences in background structure of the mediastinal organs; namely, the comparatively left location of the descending aorta and the thoracic duct, and the comparatively right situation of the membranous portion of the trachea. The morphological variations of the lymphatic structure in each specimen were greater than expected.
Schematic representations of specimens. Difference between the right and the left lymphatic drainage systems and rather great morphological variations in extramural lymphatics through specimens were noted. A Thoracic paraaortic node, AC thoracic paraaortic lymph chain, Az azygos vein, B bifurcational node, BA broncheal artery, BCV brachiocephalic vein, C cervical paratracheal node, D-Ao descending aorta, E paraesophageal node, EJV external jugular vein, Eso esophagus, IJV internal jugular vein, J deep cervical node, l left, M main bronchus node, P thoracic paratracheal node, PM posterior mediastinal node, R retrotracheal node, r right, RLN recurrent laryngeal node, SCV subclavian vein, TD thoracic duct, U uppermost thoracic paratracheal node, VA venous angle
Right lymphatic drainage system (RDS)
The background supportive structure of the lymphatic pathways from the right wall of the thoracic esophagus was the right esophageal wall itself, the membranous portion of the trachea and the right tracheal wall. The upward lymphatic flow from the right wall of the thoracic esophagus ran mainly along the membranous portion of the trachea and the right tracheal wall in specimens 1–4; and mainly along the right esophageal wall in specimen 5 (Fig. 6). The basic structure of the right lymphatic pathway was multi-stationed; more than one lymph node was intercalated between the esophagus and the right venous angle.
Left lymphatic drainage system (LDS)
The background supportive structure of the lymphatic pathways from the left wall of the thoracic esophagus was the descending aorta and the left tracheal wall. In all of the specimens, the thoracic duct was the direct drainage site from the left thoracic esophageal wall (Fig. 6). Moreover, in all specimens except specimen 4, thoracic duct was the main lymphatic drainage site from the left thoracic esophageal wall. An ascending lymphatic pathway along the left tracheal wall from the left thoracic esophageal wall was observed only in specimen 4 (Figs. 4, 6).
Contralateral lymphatic flow from the thoracic esophagus
In specimens 2, 3 and 5, drainage lymphatics from the right wall of the lower thoracic esophagus ran into the LDS and terminated in the thoracic duct intercalated by several nodes (Figs. 2, 3, 5). In specimens 2 and 5, the left wall of the lower to middle thoracic esophagus drained into the RDS (Figs. 2, 5). In this way, lymphatics from the lower to middle thoracic esophagus were drained contralaterally, much more frequently than expected.
Ascending lymphatic connection
It was notable that, in all specimens, the right uppermost paratracheal nodes (U) received lymphatics from all levels of the RDS. In this respect, U were thought to be the key nodes of the thoracic esophagus. The right thoracic paratracheal nodes (P) received lymphatics from rather wide area of the thoracic esophagus, especially from the lower and the middle thoracic esophagus. However, right P did not receive lymphatics from the upper and uppermost thoracic esophagus, except in specimens 2 and 4.
In specimens 1 and 5, lymphatics of the RDS did not ascend to reach the cervical paratracheal nodes (C) or deep cervical nodes (J). However, in specimens 2–4, lymphatics from the RDS ascended to the right C or J by way of U or P. These findings indicate that not only U or P, but also C or J could function as the final lymphatic station from the thoracic esophagus, before emptying into the right venous angle.
The RDS was basically an ascending lymphatic pathway along the trachea or the esophageal wall. In the LDS, the ascending lymphatic pathway along the left tracheal wall was not so well developed compared to the RDS except in specimen 4. However, the left lower to middle thoracic esophageal wall drained into the RDS via a contralateral connection in specimens 2 and 5.
First lymphatic drainage site or the anatomical sentinel node (SN) from each level of the thoracic esophagus
In the RDS, the paraesophageal node, bifurcational node, posterior mediastinal node and main bronchus node could be the SN of the lower and middle thoracic esophagus. The tracheobroncheal node, retrotracheal node, thoracic paratracheal node and right uppermost paratracheal node could be the SN of the upper and uppermost thoracic esophagus.
In the LDS, paraesophageal node, the posterior mediastinal node and the thoracic paraaortic node could be the SN of the lower and middle thoracic esophagus, and thoracic paratracheal node could be the SN of the upper and uppermost thoracic esophagus. However, in the LDS, it was consistently observed that the thoracic duct was the first drainage site from any level of the thoracic esophagus, as mentioned above.
Discussion
Anatomical summary and considerations of our specimens
We have shown commonly observed facts through our five specimens: difference of the right and left lymphatic drainage systems with rather greatly varied topographic structures in each specimen. All of our donors were more than 70 years of age at death (Table 1). It has been described that the lymphatic systems atrophy with age [22]. Atrophy of the lymphatic systems might not serve the clear identification of the lymphatic pathways. In this sense, clearly observed facts described above could be valuable even with a small number of specimens, and might serve general surgical principles for the thoracic esophageal cancers.
Intramural and extramural lymphatics from the thoracic esophagus and its ascending or descending flow
The intramural lymphatics of the esophagus have been anatomically classified to a mucous and a muscular [22, 24]. The collecting trunks originate in the submucous tissue and are disposed in two different ways: some traverse the muscularis at once and empty in the nearest nodes; the others ascend or descend vertically beneath the mucosa for some distance before traversing the muscularis; those coming from the upper two-thirds of the esophagus ascend, while the trunks from the lower third descend [22, 24].
Natsugoe investigated the intramural and extramural lymphatic flow from the distal esophagus and gastric cardia of mongrel dogs with a dye injection method [18], and showed predominant ascending flow in the mucosal layer and predominant descending flow in the submucosal layer or deeper in this region. He also described both ascending or descending extramural flow from the distal esophagus, and predominant descending flow from the gastric cardia [18].
Tanabe et al. described the lymphatic flow from the thoracic esophagus in their lymphoscintigraphical study, with preoperative injection of the 99mTc Renium Colloid into the esophageal wall, as follows: from the upper third thoracic esophagus, to the neck and upper mediastinum; from the middle third, to the neck, upper mediastinum and the abdomen; from the lower third, mainly to the abdomen with less predominant ascending flow [28]. They also showed rich flow to the thoracic paratracheal nodes and supraclavicular nodes from the upper and middle thoracic esophagus [28].
Cervical through abdominal lymph node metastases have been observed in thoracic esophageal cancers [4, 5, 14–16, 19, 26, 27, 29]. It has been proved clinicopathologically by these findings, that both ascending and descending lymphatic flow is possible from the thoracic esophagus.
Frequency of metastasis to the right and left recurrent nerve nodes from thoracic esophageal cancer
Fujita et al. reported that the right recurrent nerve, right paracardiac, periesophageal and lesser curvature nodes were the most frequent metastastic nodes in three-field dissection of thoracic esophageal cancer (TEC) [4]. They showed a metastatic rate in the right and left recurrent nerve nodes (RN) of 30.0 and 21.4%, respectively [4]. Sharma et al. [26] reported that the right recurrent nerve and paracardiac nodes were the most frequent metastatic nodes in TEC. Their data on the metastatic rate in the right and left RN were 34.3 and 10.0%, respectively [26]. Similar result was reported by Isono et al. [5] in the cumulative data of the TEC in Japan. The present data support their result on the predominance of the right RN to the left RN as the metastatic site, because the uppermost paratracheal node received lymphatic vessels from all levels of the right drainage system, and relatively poor development of the left paratracheal drainage pathway was found in our specimens.
Frequency of metastasis to bifucational nodes from the lower and middle thoracic esophagus
Our specimens showed rather great variations in the development of the lymphatic drainage systems as mentioned above. However, extramural lymphatic vessels to bifurcational nodes from the lower and middle thoracic esophagus were relatively consistently observed through all specimens (Fig. 6). Isono et al. [5] reported the metastatic rate to bifurcational nodes from the lower and middle TEC to be 12.3 and 16.7%, respectively, which was higher than other peritracheal lymph node groups.
Thoracic paraaortic nodal metastasis
No literature was found which dealt with thoracic paraaortic nodal metastasis from squamous cell carcinoma (SCC) of the thoracic esophagus. This might be because the thoracic paraaortic nodes are classified as the posterior mediastinal nodes [7]. Dresner et al. [3] showed a different pattern of nodal metastases from adenocarcinoma of the esophagogastric junction compared with SCC of the thoracic esophagus. They reported relatively frequent paraaortic nodal metastasis from adenocarcinoma of the distal esophagus [3].
Initial lymph node metastasis from thoracic esophageal cancer
Matsubara et al. reported that the right recurrent nerve and paracardiac nodes were the most frequent metastatic nodes in superficial carcinoma of the thoracic esophagus regardless of original site [15]. They also described a similar result in initial lymph node metastasis from thoracic esophageal carcinoma and stressed the importance of intramural longitudinal lymphatic spread [16]. Natsugoe et al. described the initial lymph node metastasis including micrometastasis from TEC [19]. From their data of superficial TEC, lymph node metastasis was confined to recurrent nerve nodes from the upper thoracic esophagus; and recurrent nerve, paraesophageal or perigastric nodes from the lower and middle thoracic esophagus [19]. Although their data do not necessarily contradict ours, the initial metastatic frequency to bifurcational nodes from the middle and lower TEC could be a little higher considering our data.
This might require esophageal intramural lymphatic development for explanation. The mucosal lymph capillary network of the esophagus is continuous to the pharynx or stomach without a distinct demarcation [22]. The mucosal and submucosal lymph capillaries communicate freely in the long axis; however, they are limited circumferentially [31]. Some of the collecting trunks, which drain the mucosal and submucosal lymphatics, ascend or descend vertically before traversing the muscularis [22, 24]. These anatomical structures might cause lymph node metastasis at a considerable distance. In this argument, the bifurcational node might be overpassed to the recurrent nerve node as an initial metastatic node, from the middle and lower TEC in its relatively early stage of disease. Single node metastasis in the neck from gastroesophageal junction adenocarcinoma without involved thoracic nodes, has been reported [30]. This might also require wide-range vertical intramural lymphatic spread before extramural drainage as an explanation.
Risk factor of hematogenous and lymphatic spread from thoracic esophageal cancer
As for the factors concerned with survival after esophagectomy with three-field lymphadenectomy, the grade of lymph node metastasis was reported to be more closely related than the depth of invasion [27]. Lymphatic invasion or high numbers of lymph node metastasis has been proven to be a risk factor for hematogenous recurrence [9, 21, 27]. Our study has proved that the thoracic duct could often be the initial lymphatic drainage site in the LDS. This means that hematogenous metastasis from thoracic esophageal cancer via the thoracic duct is possible when the tumor invades the lymphatic vessels. It could be assumed that hematogenous metastasis is possible even at an early stage like submucosal invasion of tumor, because lymph node metastasis is possible at this level of tumor invasion [15].
However, Izbicki et al. [6] reported in their immunohistochemical study, that the presence of micrometastatic tumor cells in bone marrow had no additional prognostic value, suggesting that the main prognostic factor in esophageal cancer is the spread of the tumor to the lymph nodes; hematogenous spread may be a secondary event.
Clinical studies have proven the improvement of prognosis after three-field compared with two-field lymph node dissection in esophagectomy [1, 5, 29]. If the hematogenous spread was a predominant factor for prognosis after esophagectomy, three-field dissection could only have a small impact on the prognosis.
Clinicopathologically proven risk factors concerned with hematogenous spread, other than the lymph node metastasis, are reported as follows: high microvessel density [12], negative E-cadherin expression [9]. Hyperlipidemia is reported to be a risk factor for lymphatic metastasis in superficial esophageal carcinoma [25]. It is interesting that the preoperative C-reactive protein could be an indicator of prognosis in esophageal carcinoma [20].
Sentinel nodes (SN) of the thoracic esophagus
The sentinel node (SN) is defined as the first lymph node in a regional lymphatic basin that receives lymph flow from a primary tumor [8]. The sentinel node concept has been advocated for melanoma and breast cancer in the past decade [11]. Recently, sentinel node navigation surgery has been adopted in gastrointestinal cancer surgery [10]. Some institutions have reported promising outcomes with high accuracy of the SN in thoracic esophageal cancer surgery [8, 13].
The basic assumption of lymphatic metastasis will be as follows: individual topographical lymphatic anatomy and oncological behavior of the tumor are associated with lymph node metastasis. Kitagawa et al. suggested that SN could be more than one and the SN may be both patient- and lesion-specific in gastrointestinal cancer [11].
Although clinicopathological studies have revealed frequent initial metastasis to recurrent nerve nodes and paracardiac nodes in relatively early-stage thoracic esophageal cancer, lymph node metastasis is not confined to these two groups. Considering the lymphatic anatomical variation in each specimen of our study, minimally invasive sentinel node navigation surgery for thoracic esophageal cancers would be feasible with careful identification of SNs in application of the gamma probe, at least in the early-phase disease. However, adjuvant therapy should be planned with pathological assessment of the oncological nature of the tumor just as it has been after conventional treatment.
References
Altorki N, Kent M, Ferrara C et al (2002) Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 236:177–183
Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ (1995) Gray’s anatomy, 38th edn. Churchill Livingstone, New York, pp 1605–1626
Dresner SM, Lamb PJ, Bennet MK et al (2001) The pattern of metastatic lymph node dissemination from adenocarcinoma of the thoracic esophagogastric junction. Surgery 129:103–109
Fujita H, Kakegawa T, Yamana H et al (1994) Lymph node metastasis and recurrence in patients with a carcinoma of the thoracic esophagus who underwent three-field dissection. World J Surg 18:266–272
Isono K, Sato H, Nakayama K (1991) Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 48:411–420
Izbicki JR, Hosch SB, Pichlmeier U et al (1997) Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med 337:1188–1194
Japanese Society for Esophageal Diseases (1999) Guidelines for the clinical and pathologic studies on carcinoma of the esophagus, 9th edn. Kanehara, Tokyo
Kato H, Miyazaki T, Nakajima M et al (2003) Sentinel lymph nodes with Technetium-99m colloidal rhenium sulfide in patients with esophageal carcinoma. Cancer 98:932–939
Kato H, Miyazaki T, Nakajima M et al (2003) Prediction of hematogenous recurrence in patients with esophageal carcinoma. Jpn J Thorac Cardiovasc Surg 51:599–608
Kitagawa Y, Ohgami M, Fujii H (2001) Laparoscopic detection of sentinel lymph nodes in gastrointestinal cancer: a novel and minimally invasive approach. Ann Surg Oncol 8:86S–89S
Kitagawa Y, Kitajima M (2002) Gastrointestinal cancer and sentinel node navigation surgery. J Surg Oncol 79:188–193
Kyriazanos ID, Tachibana M, Shibakita M et al (2003) Pattern of recurrence after extended esophagectomy for squamous cell carcinoma of the esophagus. Hepatogastroenterology 50:115–120
Lamb PJ, Griffin SM, Burt AD et al (2005) Sentinel node biopsy to evaluate the metastatic dissemination of esophageal adenocarcinoma. Br J Surg 92:60–67
Matsubara T, Ueda M, Yanagida O et al (1994) How extensive should lymph node dissection be for cancer of the thoracic esophagus? J Thorac Cardiovasc Surg 107:1073–1078
Matsubara T, Ueda M, Abe T et al (1999) Unique distribution patterns of metastatic lymph nodes in patients with superficial carcinoma of the thoracic esophagus. Br J Surg 86:669–673
Matsubara T, Ueda M, Kaisaki S et al (2000) Localization of initial lymph node metastasis from carcinoma of the thoracic esophagus. Cancer 89:1869–1873
Nakagawa S, Kanda T, Kosugi S et al (2004) Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 198:205–211
Natsugoe S (1989) Experimental and clinical study on the lymphatic pathway draining from the distal esophagus and gastric cardia (in Japanese with English abstract). J Jpn Surg Soc (Nippon Geka Gakkai Zasshi) 90:364–376
Natsugoe S, Matsumoto M, Okumura H et al (2005) Initial metastatic, including micrometastatic, sites of lymph nodes in esophageal squamous cell carcinoma. J Surg Oncol 89:6–11
Nozoe T, Saeki H, Sugimachi K (2001) Significance of preopertive elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg 182:197–201
Osugi H, Takemura M, Takada N et al (2002) Prognostic factors after oesophagectomy and extended lymphadenectomy for squamous oesophageal cancer. Br J Surg 89:909–913
Rouvière H (1938) Anatomy of the human lymphatic system. Edwards Brothers, Ann Arbor, pp 5–62
Saito H, Sato T, Yamashita Y et al (2002) Topographical analysis of lymphatic pathways from the meso- and hypopharynx based on minute cadaveric dissections: possible application to neck dissection in pharyngeal cancer surgery. Surg Radiol Anat 24:38–49
Sakata K (1903) Ueber die Lymphgefäße des Oesophagus and über seine regionären Lymphdrüsen mit Berücksichtigung der Verbreitung des Carcinoms. Mitt Grenzgeb Med Chir 11:634–656
Sako A, Kitayama J, Kaisaki S et al (2004) Hyperlipidemia is a risk factor for lymphatic metastasis in superficial esophageal carcinoma. Cancer Lett 208:43–49
Sharma S, Fujita H, Yamana H et al (1994) Patterns of lymph node metastasis in 3-field dissection for carcinoma in the thoracic esophagus. Surg Today 24:410–414
Takemura M, Osugi H, Takada N et al (2003) Prognostic factors in patients with squamous esophageal cancer associated with solitary lymph node metastasis after oesophagectomy and extended lymphadenectomy. Oncol Rep 10:75–80
Tanabe G, Baba M, Kuroshima K et al (1986) Clinical evaluation of esophageal lymph flow system based on the RI uptake of removed regional lymph nodes following lymphoscintigraphy. (in Japanese with English abstract). J Jpn Surg Soc (Nippon Geka Gakkai Zasshi) 16:315–323
Tsurumaru M, Kajiyama Y, Udagawa H et al (2001) Outcomes of extended lymph node dissection for squamous cell carcinoma of the thoracic esophagus. Ann Thorac Cardiovasc Surg 7:325–329
van de Ven C, De Leyn P, Coosemans W et al (1999) Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur J Cardiothorac Surg 15:769–733
Weinberg JA (1972) Lymphatics of the esophagus. In: Haagensen CD, Feind CR, Herter FP, Slanetz CA Jr, Weinberg JA (eds) The lymphatics in cancer. WB Saunders, Philadelphia, pp 245–249
Acknowledgments
We appreciate for the kind offering of the cadavers for our investigations by the Functional Anatomy of Tokyo Medical and Dental University, graduate school. All the experiments performed here comply with the current laws in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, H., Sato, T. & Miyazaki, M. Extramural lymphatic drainage from the thoracic esophagus based on minute cadaveric dissections: fundamentals for the sentinel node navigation surgery for the thoracic esophageal cancers. Surg Radiol Anat 29, 531–542 (2007). https://doi.org/10.1007/s00276-007-0257-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-007-0257-6