Abstract
In view of the paucity of literature, this study was undertaken to reappraise the gross anatomy of the sacrotuberous ligament (STL), with the objective of providing an accurate anatomical basis for clinical conditions involving the STL. We studied the gross anatomy of the STL in 50 formalin fixed cadavers (100 sides) during the period of 2004–2005. All specimens exhibited an STL with a ligamentous part and (87%) of specimens exhibited a membranous (falciform) segment, which extended towards the ischioanal fossa. The variations of the falciform extensions were classified into three types. In Type I (69%), the falciform process extended towards and along the ischial ramus to terminate at the obturator fascia. In Type II (108%), the falciform process extended along the ischial ramus, fused with the obturator fascia and continued towards the ischioanal fossa. In addition, the medial border of the falciform process descended to fuse with the anococcygeal ligament, forming a continuous membrane. Lastly, in Type III (13%), the falciform process of the STL was absent. The above mentioned data could have an important implication to the understanding of the relationship between the pudendal nerve and the sacrotuberous ligament and their relevance to pudendal nerve entrapment syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sacrotuberous ligament (STL) is a strong supporting ligament that provides stability to the sacroiliac region (accessory to sacrospinous ligament), by counterbalancing its rotation, therefore limiting the movement of the lower portion of the sacrum [9]. This becomes very important in weight transmission, when the vertebral column sustains considerable force, possibly due to the attachments of the STL. The STL attaches broadly to the posterior superior iliac spine (PSIS), the transverse tubercles of the sacrum and the upper part of the coccyx. Its oblique fibers converge and descend laterally to attach to the medial surface of the ischial tuberosity. This attachment widens slightly and curves along the ramus of the ischium to form the falciform process [18]. Some of the lower fibers of the gluteus maximus originate from the posterior surface of the STL [18]. The STL is thought to originate as the degenerated tendon of the long head of the biceps femoris and some of its fibers still continue into the tendon [16]. However, to the best of our knowledge there is little information in the literature, which describes the variations in origin, insertion and other attachments of the STL. According to Antolak et al. [1], more attention must be paid to the transverse diameter of the STL in order to better understand its role in pudendal nerve entrapment.
The clinical significance of the STL has been given a limited attention in anatomical and surgical textbooks. However, in recent studies, the ligament has been cited in its association with pudendal nerve entrapment syndrome [11]. The pudendal nerve (S2, S3, S4) is the principal sensory nerve of the external genitalia and the perineum and its entrapment is a known cause of chronic perineal pain [15]. According to Hough et al. [5], the pudendal nerve enters Alcock’s (pudendal) canal, which is formed by duplication of the obturator fascia, inferior to the falciform process and the insertion of the STL into the ischial tuberosity. It is for this reason that pudendal nerve entrapment often results in pain or loss of sensation in the penis, scrotum, labia, perineum or rectum [5]. Fecal incontinence can also result from compression of the pudendal nerve [14]. Robert et al. [11], suggested that there are three possible sites of pudendal nerve constriction: between the sacrotuberous and sacrospinous ligaments, in the pudendal canal and by the falciform process of the STL [11].
The question could be raised therefore, as to what degree the anatomy of the STL is involved in the etiology of pudendal nerve entrapment syndrome. The aim of our study was to describe the morphology of the STL, including possible variations in the origin, attachments and thickness of the ligament.
Materials and methods
We examined 50 (100 sides) adult human cadavers during the anatomy course at the American University of the Caribbean, School of Medicine throughout the academic semesters of 2003–2004. The cadavers were those of 15 female and 35 male subjects with an age range of 65–74 years and a mean age of 67 years. All the cadavers were routinely fixed in formalin–phenol–alcohol solution. None of the cadavers revealed any evidence of previous surgical procedures or traumatic lesions to the perineal or gluteal regions.
Following the preliminary examination, images from all dissected specimens were recorded with a Sony digital camera (model: Sony Cyber-Shot DSC-f717) and studied using a computer-assisted image analysis system (all measurements were carried out with the Lucia program [1998 edition for Windows], made by Nikon [Laboratory Imaging Ltd., Precoptic Co., Medical and Optical Instruments, Poland]). The digital camera was connected to an image processor (Nvidia Riva TNT model 64) with linkage to a mainframe computer. Digitized images of the STL, together with their surrounding structures, were stored in the Lucia program, (1,152×864 pixels) and converted to intensity gray levels from 0 (darkest) to 32 bit (lightest). After applying a standard 1 mm scale to all pictures within the program, Lucia was able to use this information to calculate pixel differences between two selected points (example origin–termination) of the STL. The purpose of the software was to allow easy and accurate translation of pixel differences into metric measurements, as previously described [7].
Specifically, the distance was measured from the origin of the STL (from the PSIS) to its termination (midline of insertion upon ischial tuberosity). The thickness was measured at the midpoint of the ligamentous portion. The width of STL was measured at three points: proximal (defined as the widest point of origin), middle (the midpoint of the ligamentous portion of STL) and distal (the widest point of attachment to the ischial tuberosity, excluding the falciform portion). In addition, dimensions of the falciform portion of the STL were also measured. The length of the falciform portion was measured from the medial border of the ligamentous portion of STL to its termination. In order to differentiate between obturator fascia and falciform process, the obturator fascia was exposed and separated from the falciform process until a point at which no further separation was possible. For cases, which the falciform process continued beyond the obturator fascia, the two were again separated distally in order to determine that the continuation along the ischioanal fossa was derived from the falciform process and not from the obturator fascia.
Results
We observed a bilateral occurrence of the STL in 50 (100%) specimens. The dissections revealed that the STL was composed of two distinct parts: a tough ligamentous band and a membranous falciform process.
All the specimens exhibited the ligamentous part of the STL, which was broadly attached by its base to the PSIS. The attachments of the STL included fusion with the posterior sacroiliac ligaments, the lower transverse ligaments, the lower transverse sacral tubercles, the inferior margins sacrum and the upper coccyx. The length, from origin to insertion, ranged from 65 to 121 mm with a mean of 86 mm. The mean thickness was 4 mm with a range of 2.5 to 5.8 mm. The proximal width of the STL, ranged from 52 to 95 mm with a mean of 76 mm. The middle width ranged from 22 to 45 mm with a mean of 35 mm, while the distal width ranged from 42 to 75 mm with a mean of 58 mm. These dimensions contributed to the formation of wide or narrow sciatic foramina. In 10 (10%) specimens, ligamentous fibers from the sacroiliac joint fused with the STL, forming a continuous structure, such that we were unable to distinguish the exact borders of these two ligaments.
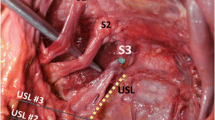
Eighty-seven (87%) of the specimens also had a membranous, non-ligamentous portion of the STL (falciform process) that extended towards the ischioanal fossa (Figs. 1, 2). However, variations existed, in the degree of the attachment of the falciform process to the ischial ramus. In order to facilitate comparisons between the falciform processes of the specimens and to analyze the extent of their attachment, the results of the dissections were classified into three types (Figs. 3, 4, 5; Table 1).
The most common form of STL was Type I (69, 69%), in which the falciform process extended towards and along the ischial ramus to terminate at the obturator fascia (Fig. 3). The length of the falciform process ranged from 12 to 20 mm with a mean of 16 mm. Type II occurred in 18 (18%) of the specimens. The falciform process extended along the ischial ramus, fused with the obturator fascia and continued to the ischioanal fossa (Fig. 4). In addition, the medial border of the falciform process of the STL descended to fuse with the lateral anococcygeal ligament, forming a continuous membrane involving three structures: the STL, the obturator fascia and the anococcygeal ligament. This type exhibited a falciform process with a length ranging from 35 to 54 mm and a mean of 46 mm. Lastly, in Type III (13, 13%), the falciform process of the STL was absent (Figs. 5, 6). The attachment of this type was limited to the ischial tuberosity and there was no extension of the ligament along the ischial ramus.
All the STLs contained a coccygeal branch of the inferior gluteal artery. The coccygeal branch passed posterior to the midportion of the sacrospinous ligament to pierce the STL at multiple sites.
The pudendal nerve and internal pudendal arteries were found in all the specimens, to be enclosed by a fascial sheath. In Type III specimens, in which no falciform process was present, the covering of Alcock’s canal was composed solely of fascia derived from the obturator internus. Accordingly, this type appeared to allow the most space for the nerves and vessels within the canal. With increasing thickness of the overlying fascia, we observed a corresponding decrease in the free space within the canal. For instance, in Type I specimens, the fusion of the falciform process with the obturator fascia, led to an increase in the thickness of the overlying fascia. Furthermore, the fascial sheath with greatest thickness was observed to occur in Type II specimens. Accordingly, it was this type, which was observed to have the least free space for the nerves and vessels occupying the canal. Although, detailed topographical description of the pudendal nerve is beyond the scope of our study, it would be interesting to compare the relationships of pudendal nerve topography with the various types of STL.
With regards to symmetry of the STL patterns, it was noted that Type I, II, III appeared to be symmetrical in 25 (25%), 5 (5%) and 3 (3%) of the cases, respectively. No differences were noticed in STL morphometry or topography with respect to race, age or gender.
Discussion
In their anatomical descriptions of the STLs, standard anatomical textbooks [3, 18] give only a brief definition of the STL, without providing information regarding anatomy of the falciform process or its dimensions. To the best of our knowledge, this study of 50 cadavers is the first to present the dimensional anatomy of the STL and a classification of its types in relation to the falciform process. In view of the fact that little information on the STL exists in anatomical textbooks and that recent studies have implicated the ligament as a possible cause of pudendal nerve entrapment, it appeared necessary to provide a more complete description the STL and particularly the falciform process.
One previous study by Prescher and Bohndorf [10] provided measurements of the STL length as ranging from 1.1 to 7.2 cm. This is significantly different from our findings of STL length, as we observed no specimens in which the STL was as short as 1.1 cm. We are unable to determine whether this discrepancy was due to the difference in measurement technique or population from which the specimens were derived. In addition, that study does not make any mention to measurements of the falciform process of the STL.
In contrast to descriptions of the aforementioned authors, Barnes [2], Derry [4] and Smith [17] state that it is not correct to describe Alcock’s canal as formed by the “splitting of the obturator fascia.” It consists of an investment of fascia (fibrous sheath), which has nothing to do with the sheath of the obturator internus muscle and is attached to the neighboring bony and ligamentous structures quite independently. It often happens that this fascia (lunate fascia) becomes attached to the surface of the fascia of the obturator internus but this does not always happen. Our results are consistent with previous description in older anatomical texts [2, 4, 17], which indicate that the lunate fascia is actually the falciform process of the STL, which variably contributes to Alcock’s canal.
Pudendal nerve entrapment and the resulting neuropathy have been implicated as the cause of several clinical syndromes. Townsend reported the presence of pudendal neuropathy in 70% of patients with fecal incontinence [19]. Additional reports by Mauillon et al. [8] and Antolak et al. [1] have described pudendal neuropathy in patients with symptoms varying from chronic perineal pain to anorectal anesthesia. In fact, Roberts et al. [12], report that 95% of patients with confirmed diagnosis of chronic prostatitis have no evidence of bacterial infection or inflammatory cells. The potential consequences of misdiagnosis can include: prolongation of nerve trauma and inappropriate surgical treatment. Clinically, the picture may be further complicated by the fact that radiologists are unable to accurately diagnose pudendal nerve entrapment with MRI or CT [6]. Furthermore, damage to the pudendal nerve has also been implicated in the etiology of erectile dysfunction [13]. Considering the wide array of clinical problems resulting from pudendal neuropathy, knowledge of the possible anatomical variations of the STL could prove useful to both anatomists and surgeons alike.
Several studies have suggested the involvement of the STL in pudendal nerve entrapment syndrome. According to Robert et al. [11], the STL and sacrospinous ligaments can act like a ‘lobster claw,’ with the pudendal nerve traversing the interligamanentous space, where it can be crushed. However, there seems to be no description of the falciform process and its fusion to the obturator fascia, which together may form Alcock’s canal.
A study by Hough et al. [5] examined pudendal nerve entrapment as a cause of chronic perineal pain. In their study, Hough et al. [5] mention the possibility that the pudendal nerve can be ensheathed by ligamentous expansions, forming a perineural compartment. Our study has shown that, in Type II specimens, these fascial attachments were tightly adherent and difficult to detach, resulting in considerable narrowing of the space, lateral to the pudendal nerve. Hough et al. [5], also describe the existence of a thickened area of duplication of the obturator fascia, which may also act as an entrapment site for the pudendal nerve. Although, our study did not include histological analysis, the present authors propose that this thickened membranous layer was not a duplication of the obturator fascia but rather the falciform process of the STL. This determination is based upon our experience from careful dissection as described previously. An interesting follow-up study would include ultrastructural and histological examination to determine the nature of these fibers. Based on the degree of thickening and the resultant narrowing of the pudendal canal, it is possible that the presence of STL Type II may particularly predispose patients to pudendal nerve entrapment at this site. Furthermore, although the falciform process is absent in type III, it is still possible that the pudendal nerve may become entrapped by the thickening of the obturator fascia.
Another possible site of pudendal nerve entrapment involves all types of STL. As the pudendal nerve exits the lesser sciatic foramen and courses under the STL, the fibers of the fascial sheath fuse with the STL. This fusion and thickening of fibers combined with a curve of the pudendal nerve to enter the perineum could contribute to compression in this location. This is particularly important with patients exhibiting larger middle and distal widths of the ligamentous portion of the STL. In addition, according to Antolak et al. [1], the gluteus muscle is intimately attached to the STL and may exert a shearing effect as it extends the hip.
Pudendal neuropathy has also been hypothesized to be the result of increased traction on the nerve within Alcock’s canal [13]. The presence of a falciform process, as described in types I and II, could possibly increase the degree of nerve traction as the pelvic floor is forced inferiorly during the Valsalva maneuver.
By providing a new data regarding the topography and morphometry of the STL, we hope to provide anatomists and surgeons with a better understanding of the anatomical basis of pudendal nerve entrapment. It is possible that this knowledge may not only play a role in the diagnosis, but also in the development of new treatments for this common clinical problem.
References
Antolak SJ, Hough DM, Pawlina W, Spinner RJ (2002) Anatomical basis of chronic pelvic pain syndrome: the ischial spine and pudendal nerve entrapment. Med Hypotheses 59:349–353
Barnes AR (1921) Pelvic fascia. Anat Rec 21:36–55
Clemente CD (1985) Gray’s anatomy—American edition. Williams and Wilkins, Baltimore, pp 724–726
Derry DE (1907) On the real nature of the so-called pelvic fascia. J Anat Physiol 42:95–106
Hough DM, Wittenberg KH, Pawlina W, Maus TP, King BF, Vrtiska TJ, Farrell MA, Antolak SJ Jr (2003) Chronic perineal pain caused by pudendal nerve entrapment: anatomy and CT-guided perineural injection technique. AJR 181:561–567
Kawanishi Y, Lee KS, Kimura K, Kojima K, Yamamoto A, Numata A (2001) Feasabilty of multi-slice computed tomography in the diagnosis of arteriogenic erectile dysfunction. BJU Intl 88:390–395
Loukas M, Hullett J, Wagner T (2005) The clinical anatomy of the inferior phrenic artery. Clin Anat 18:357–365
Mauillon J, Thoumas D, Leroi AM, Freger P, Michot F, Denis P (1999) Results of pudendal nerve neurolysis-transposition in twelve patients suffering from pudendal neuralgia. Dis Colon Rectum 42:186–192
Moore KL, Dalley AF (1999) Clinically oriented anatomy. 4th edn. Lippincott Willimas & Wilkins, Philadelphia, pp 340–341
Prescher A, Bohndorf K (1993) Anatomical and radiological observations concerning ossification of the sacrotuberous ligament: is there a relation to spinal diffuse idiopathic skeletal hyperostosis (DISH)? Skeletal Radiol 22:581–585
Robert R, Prat-Pradal D, Labat JJ, Bensignor M, Raoul S, Rebai R, Leborgne J (1998) Anatomic basis of chronic perianal pain: role of the pudendal nerve. Surg Radiol Anat 20:93–98
Roberts RO, Lieber MM, Bostwick DG, Jacobsen SJ. (1997) A review of clinical and pathological prostatits syndromes. Urology 49:809–821
Shafik A (1994) Pudendal canal decompression in the treatment of erectile dysfunction. Arch Androl 32:141–149
Shafik A (1997) Role of pudendal canal syndrome in the etiology of fecal incontinence in rectal prolapse. Digestion 58:489–493
Shafik A, el-Sherif M, Youssef A, Olfat ES (1995) Surgical anatomy of the pudendal nerve and its clinical implications. Clin Anat 8:110–115
Sinnatamby CS (1999) Last’s anatomy regional and applied. 10th edn. Churchill Livingstone, Edinburgh, pp 315–316
Smith GE (1908) Studies in the anatomy of the pelvis, with special reference to the fasciae and visceral supports. J Anat Physiol 42:198–218
Standring S, Ellis H, Healy C, Johnson D, Williams A (eds) (2005) Gray’s Anatomy Elsevier Churchill Livingstone, pp 1428, 1439
Townsend CM (2001) Sabiston textbook of surgery: the biological basis of modern surgical practice. WB Saunders. Philadelphia, pp 939–940
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loukas, M., Louis, R.G., Hallner, B. et al. Anatomical and surgical considerations of the sacrotuberous ligament and its relevance in pudendal nerve entrapment syndrome. Surg Radiol Anat 28, 163–169 (2006). https://doi.org/10.1007/s00276-006-0082-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-006-0082-3