Abstract
Irrigation waters have come under increasing scrutiny as a potential source of pathogenic microorganisms contaminating fresh produce. It is generally assumed that the microbial concentrations entering and leaving irrigation pipe networks are identical. However, this may not be true if biofilms form on the inner surfaces of irrigation pipes. The retention and release of pathogens in biofilms are well documented in drinking water distribution systems, but very little data are available for irrigation systems. We examined the attachment and/or incorporation of total coliform, fecal coliform, and Escherichia coli bacteria into biofilms in new and used aluminum irrigation pipe. Water from a local creek in Maryland was used to conduct weekly irrigation events. Prior to each event, removable sections of pipe (coupons) were scraped to determine the extent of bacterial attachment; in addition, bacterial concentrations in residual water were determined. Substantial populations of coliform bacteria were found on the pipe surfaces. Old pipes had fewer attached or biofilm-incorporated coliforms and lower coliform concentrations in the residual water. High probabilities were found for average fecal coliform and total coliform concentrations being different between creek water and sprinkler water. These results have implications for monitoring and control of microbial quality of irrigation waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Produce has been implicated in several food-related illness outbreaks (US CDC 2012). Because produce is frequently grown under irrigation, the microbiological quality of irrigation waters has come under greater scrutiny and is the subject of regulation (Gerba et al. 2009; Pachepsky et al. 2011). Previous research has shown that pathogens from irrigation water can survive within leaf and other plant tissues (Brandl 2006),
The microbiological quality of water in drinking water delivery systems has long been known to be affected by biofilms on pipes (US EPA 2002a). Both pathogenic and indicator organisms have been routinely detected in biofilms in drinking systems (Berry et al. 2006). For example, Fass et al. (1996) documented that up to 50 % of E. coli strains injected into a pilot scale water distribution system became attached to the surface of the cast iron pipe, where they proliferated.
Sadovski et al. (1978) were the first to point out the possibility of changes in microbiological quality of irrigation water due to attachment and detachment of microorganisms to the organic material on walls of pipes. These authors monitored the effluent from drip irrigation lines for traceable microorganisms added to treated wastewater and observed small peaks in bacterial colony counts on days 2 and 8 and in viral counts on day 8. They hypothesized that the elution of microorganisms from the irrigation system was affected by adsorption of viruses and bacteria to the organic matter that lined the inside walls of the irrigation system; repeated irrigations may have resulted in the release of the organisms. Research devoted to an understanding of the nature of irrigation system clogging and biofouling has documented biofilm formation on the surfaces of irrigation delivery materials (Dehghanisanij et al. 2004; Li et al. 2012). However, there is no published information on pathogen and indicator organism incorporation into and survival in biofilms forming in irrigation systems, potentially creating reservoirs of pathogens that may be subsequently released from biofilms during irrigation events. Only recently, a substantial change in E. coli concentrations in water passing through irrigation pipes was reported (Pachepsky et al. 2012). High E. coli concentrations in water remaining in irrigation pipes between irrigation events were indicative of E. coli growth. In two of the four irrigations, the probability of the sample source (creek vs. sprinkler), being a noninfluential factor, was only 0.14, that is, source was an important factor. The population of bacteria associated with the biofilm on pipe walls was estimated to be larger than that in water in pipes.
The materials of pipes can affect biofilm development and water quality changes (Hallam et al. 2001; Kilb et al. 2003; Silhan et al. 2006). In drinking water systems, iron and polyethylene pipe materials were shown to stimulate biofilm growth by releasing phosphorus in bioavailable forms (Lehtola et al. 2004a; Morton et al. 2005). Corroding iron and steel can abiotically produce all of the main macronutrients necessary for heterotrophic bacterial regrowth including fixed carbon, fixed nitrogen, and phosphorus (Morton et al. 2005). Soft deposits that settle to the pipe floor can be a major source of available inorganic nutrients (Lehtola et al. 2004b). Roughness of pipe surface and corrosion products also affect bacterial attachment on the surface (Chang et al. 2003). Yu et al. (2010) indicated that the pipe materials can affect not only biofilm-forming potential but also microbial diversity characterized by bacterial 16S ribosomal DNA fragments. The highest values of phylotype richness and diversity indices were found in polybutylene pipes, and the lowest were in chlorinated polyvinyl chloride pipes (Yu et al. 2010). Irrigation water delivery systems are constructed using diverse pipe materials. However, there is no published information on the effect of the pipe corrosion and weathering on microbiological water quality and biofilms in irrigation systems.
The objective of this work was to observe and report the effect of weathering of aluminum pipes on changes in microbiological water quality (e.g., total coliform, fecal coliform, and E. coli populations) as a consequence of biofilm formation in these pipes.
Materials and methods
Research in this work included (1) laboratory research of biofilm formation and comparison of the coliform extraction methods for the aluminum pipes and (2) field research on coliform concentrations in coupled samples of intake and sprinkler water as well as in standing water left in aluminum pipes, and coliforms in biofilms on pipe coupons.
Extraction efficiency study
Methods for extracting and homogenizing biofilms for bacterial counting were evaluated prior to field irrigation testing. A mixture of heterotrophic aerobic bacteria (HB) isolated from Paint Branch Creek on the USDA ARS South Farm (Beltsville, MD) was used for generating biofilms. A fresh collection of bacteria was made for each biofilm substratum tested. In some assays, E. coli K12 MG1655, ATCC 700926 (ATCC, Manassas, VA) was also added to the stream bacterial mixture. Stream organisms were isolated by surface spreading onto R2A agar (BD, Sparks, MD) and incubated at room temperature for at least 10 days. Bacteria were harvested by washing the agar plates with a small amount of sterile 0.1 M phosphate-buffered saline (PBS), pH 7.1 (Calbiochem, Darmstadt, Germany). In order to acclimate the organisms to the biofilm medium, stream bacteria and E. coli were separately grown in synthetic creek water broth for 24 h, at room temperature with gentle reciprocal shaking prior to inoculating into biofilm chambers. Fifty milliliters of these suspensions were used as the inocula for biofilm chambers. The synthetic creek medium was derived from US EPA method 821-R-02-012 (US EPA 2002b),using the moderate hardness diluent (diluent reagents: Sigma-Aldrich, Co., St. Louis, MO) modified to include humic acid as an organic substrate (Aldrich Technical Grade, sodium salt; 39.03 % total carbon) (Sigma-Aldrich, Co., St. Louis, MO), ammonium sulfate (J.T. Baker, Phillipsburg, PA), and potassium phosphate (J.T. Baker, Phillipsburg, PA) in concentrations to approximate the carbon : nitrogen : phosphorus ratio typical of freshwater in Maryland, that is, 5:1:0.01 as derived from the water quality database (USGS 2012).
Two types of aluminum pipe were used as the substrata for generating biofilm. New aluminum pipes of 7.62 cm ID were purchased from Mid-Atlantic Irrigation Co. (Farmville, VA). Used aluminum pipes of the same diameter and length were previously stored outside open to the elements for 10 years. The extent to which the pipes were used prior to experiments is unknown. Two inch (5.08 cm) long coupons were cut from used and new pipes for laboratory experiments. Pipe sections (coupons) were prepared by cutting aluminum pipe into 2 inch sections, providing an internal surface area of 18 in2. Pipe sections were cleaned with phosphate-free soapy water, triple rinsed in tap and deionized water, disinfected with 70 % ethyl alcohol, and allowed to air dry.
Coupons were placed in a 7″ × 12″ × 16″ disinfected (1 % bleach, triple rinsed in deionized water) plastic pan. Freshly made, nonsterile synthetic creek medium was added to completely submerge coupons. Medium was circulated at a rate of 106 gph with a similarly disinfected Marineland MN404 Mini-Jet submersible circulating pump (Spectrum Brands, GA). The inoculum, either mixed species creek bacteria or E. coli, was mixed into the medium. Cleaned, disinfected coupons of a particular type were numbered and randomly (via random number table) positioned in the pan. Since each biofilm recovery method was done in triplicate and medium circulation was not homogeneous, randomizing the position of coupons in the pan sought to reduce bias of mixing and flow through the coupons. Pans were covered loosely with aluminum foil and maintained at room temperature, approximately 21 °C. Biofilms were harvested after 2–3 weeks as described. The level of medium was kept constant by the addition of sterile deionized water as needed. Pan medium was sampled weekly for planktonic E.coli/total coliforms and/or creek aerobic heterotrophs.
Biofilms were recovered from the entire inner surface of coupons in the following fashion:
Coupons were removed from the pan with an alcohol disinfected hemostat and gently dipped 3X into alcohol disinfected plastic cups containing sterile deionized water to remove loose material. The outer surface was wiped with a paper towel to remove the bulk of outer surface biofilm so that it would not be dislodged into the suspension of interior biofilm being sampled. Wiping also made the coupon easier to hold. The interior of the coupon was then wetted with a 5 mL aliquot of 0.1 M PBS plus 0.04 % Tween 80 (v/v) to loosen the biofilm. The coupon was held over the bottom plate of a sterile plastic Petri dish, and a sterile flexible scraper (Fisher Scientific, Pittsburg, PA) was used to remove the biofilm. The coupon was scraped and rinsed for 9 min and the material was collected into the Petri dish. The removed biofilm was transferred into a sterile, 50-mL polypropylene centrifuge tube (Fisher Scientific, Pittsburg, PA), and topped off to 30 mL with PBS–Tween 80. Collected biofilm was immediately placed on ice and processed as soon as possible.
Eight dispersion methods were evaluated: hand shaking for 1 min with or without glass beads (4–2-mm sterile glass beads added to the suspension), vortexing at high speed for 3, 10-s interval with or without glass beads, (modified for the polyethylene hose to include 1 min sonication in an iced water bath (Sonicor SC-40, Sonicor Instrument Corp., Copiague, NY) following vortexing), and homogenizing, on ice, for 2 min. with a submerged probe (Tekmar, Tissumizer, Cincinnati, OH) at two shear speeds: 6,300 and 17,000 rpm. In pans where E. coli were added, the Colilert-18 Quanti-tray MPN system (IDEXX, Memphis, TN) was used to enumerate E. coli and total coliforms.
Field experiment
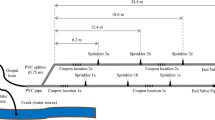
The design of two irrigation systems assembled with used and new pipes at the BARC South Farm Research Site (Beltsville, MD, USA) is shown in Fig. 1. Six inch (15.24 cm) long coupons were cut from segments of the same pipes for field experiments. A total of four coupon sets were inserted into each pipeline and were kept in place using rubber hose and stainless steel clamps over the pipe and coupon contact area. Coupons in each set were connected to each other and to the main pipe with Rain Way CL 300 clamp-on quick latch couplers. The irrigation system was placed on fairly level ground with a moderate grass cover (estimated slope of 0–2 %). Water from the nearby perennial Paint Branch Creek was used for all irrigation events.
Prior to each irrigation event (with the exception of the first irrigation), a coupon from each section was removed and a sterile plastic bag was inserted under the coupon to collect the sample of water flowing from the pipe when the coupon was unsealed (standing water). After the coupon was removed, the pipe was resealed with a rubber hose and clamps. Then, the pump was started and approximately 50 L of creek water “flushed” through the system, with the end piece removed, so as to eliminate any debris and to discard residual standing water. Afterwards, irrigation events were initiated; discharge rates for sprinklers 1, 2, and 3 were 11.2 ± 0.3, 12.0 ± 0.4 and 11.9 ± 0.5 L min−1, respectively.
Samples of creek water in triplicate and samples of water from each of the three sprinklers were taken when irrigation started (hour zero) and five minutes later, 1 h and 1 h and 5 min later, and finally 2 h and 2 h and 5 min later. All samples were stored and transported to the laboratory on ice. Water temperature inside the pipes was recorded every 5 min at the weather station located in the adjacent pump room. Three thermocouples were placed inside the pipe at each junction between sections (directly after sprinkler). The fourth thermocouple was placed on the exterior of the pipe in the first pipe section using shrink wrap.
The first irrigation event occurred on 7/13/11. The subsequent irrigation events were on 7/21/11 (Day 9), 7/28/11 (Day 16), 8/04/11 (Day 23), and 8/11/11 (Day 30); coupons and standing water were analyzed for all of these events. Weekly irrigations continued, but no samples were taken between 8/11/11 and 9/27/11. On 9/27/11 (Day 77) and 10/05/11 (Day 85), samples of water were taken. The first irrigation was conducted from 8 AM through 10 AM and the second from 10:15 AM through 12:15 PM. The first and the second irrigation events were conducted with old versus used pipe on alternating weeks.
Field sample analyses
Water samples were analyzed for total coliform, fecal coliform, and E. coli concentrations. Total coliforms and E. coli were enumerated using the Colilert-18 Quanti-tray MPN system with appropriate dilutions and expressed in MPN mL−1. Fecal coliforms were enumerated on MacConkey agar spread plates incubated at 44.5 °C for 24 h and expressed as colony-forming units per mL (CFU mL−1).
Surface-associated cells and potential biofilms were analyzed for E. coli, total coliforms, and fecal coliform counts in the same manner as the water samples. Surface-associated bacteria were obtained by scraping the bottom sector of the coupon inner surface that constituted 18 in2 (116 cm2). The 3 inch (7.62 cm) diameter to be scraped was marked with an indelible marker along the coupon exterior. Prior to scraping, the entire coupon was gently submerged into sterile deionized water and rinsed 3X to remove unattached or loosely attached bacteria and debris. The BD Falcon™ cell scraper (Fisher Scientific Catalog #08-771-1C) was used for scraping coupon interiors. In between scrapings, the cell scraper was washed under running water with a bristle brush, disinfected with 70 % ethanol, and rinsed with sterile deionized water. The coupon was held vertically over the bottom of a sterile plastic Petri dish. The marked 18 in2 section was wetted with a 5 mL aliquot of 0.01 M phosphate-buffered saline containing 0.04 % Tween 80 (PBS/Tween 80; v/v; Fisher Scientific Catalog #PI28328). The wetted section was repeatedly scraped, wetted, and rinsed for 9 min using a total of 30 mL PBS/Tween 80. All of the rinses were combined and transferred to a sterile 50-mL polypropylene centrifuge tube. If needed, the suspended biofilm was topped off to 30 mL with PBS/Tween 80. The tube was vortexed 3 times for 10 s bursts and then sonicated on ice for 1 min to break apart solid particles. The suspension samples were immediately analyzed for coliform bacteria as described above for water samples.
The statistical software PAST (Hallam et al. 2001) was used to estimate the probability of average coliform concentrations in creek and in sprinkler water being equal for each of the observation times in each of the four irrigation events using the two-tailed Student’s t statistic for three replicates.
Results
Biofilm coliform extraction efficiency
Results of the statistical comparison of the extraction methods are shown in Table 1 for the new pipe coupons. Using beads slightly increased the concentrations of extracted E.coli and total coliforms by about 80 % no matter whether handshaking with beads or sonication after vortexing was applied. Using sonication without beads resulted on average in 60 % more E. coli and in 20 % less total coliforms as compared with extraction without beads. Vortexing without sonication led to similar results both with and without beads. Probability of different average concentrations after sonication with and without beads was only 0.29 for E. coli and 0.84 for total coliforms (Table 1). Because the increase in concentrations caused by addition of beads was only moderate (about 0.2 log), and concentrations of E. coli in biofilm in the previous work (Pachepsky et al. 2012) were found without addition of beads, we applied the vortexing followed by sonication without addition of beads method for the field studies consistency.
Temperature
Figure 2 shows maximum, average, and minimum daily temperatures within used and new pipes at the first sprinkler position. The average temperatures were very close, whereas the maximum temperatures were higher in the used pipe on several days, probably because the external surface of this pipe was darker and reflected less radiation. Temperatures in all sprinkler positions (1, 2 and 3) within the used and new pipe were close to each other (within 1 °C).
Coliforms in biofilms and in residual water in pipes
The number of total coliforms attached to the pipe surface and/or incorporated into biofilms was highly variable, ranging from below the detection limit (approx. 102 MPN m−2) to 106 MPN m−2, or from approx. 5 to 50,000 per coupon (Table 2). In general, the number of total coliforms associated with the surface of new pipe was higher than old pipe. For example, an examination of the distribution of total coliform numbers (for all coupons and dates) reveals that total coliform concentrations exceeded 104 MPN m−2 five times versus nine times in new pipes. Conversely, total coliform concentrations were less than 104 in old pipes six times (two below detection limit) versus two times in new pipes. Substantially, fewer E. coli were detected attached to the pipe surface and/or incorporated into biofilms. E. coli were detected on only four coupons: one in old pipe and three in new pipe (Table 2). The majority of coupons were below the detection limit; that is, no E. coli were present on coupons.
Table 3 summarizes the data on total coliform and E. coli concentrations in the residual standing water in pipes at the beginning of the irrigation events (remaining from the prior week’s irrigation). In used pipes, total coliform concentrations in the residual water were consistently lower after a week of standing in pipes, in most instances dramatically lower. The opposite was observed in new pipes. In only two instances were concentrations lower in standing water after 1 week (coupon 1 on 4th and 11th August); in all other instances, the concentrations were comparable or increased substantially. E. coli were not detected in standing water in the majority of cases (below detection limit).
Changes in irrigation water concentrations
Graphs of temporal changes in creek and sprinkler concentrations are shown in Figs. 3, 4 and 5. Because the irrigation times were alternating, average concentrations were different; the average concentration in the second irrigation (10:15 AM to 12:15 PM) was almost always smaller than in the first irrigation (8–10 AM). The most frequently encountered temporal pattern was the decrease in concentrations of TC, FC, and E. coli during the first irrigation (8–10 AM) and relatively stable level during the second irrigation (10:15 AM to 12:15 PM), although exceptions occurred (Figs. 3, 4 and 5). Concentrations of total coliforms decreased as the experiment progressed. They were above the detection limit (2419 MPN mL−1) in summer and between 50 and 300 MPN mL−1 in the fall (Fig. 5). Fecal coliforms reached maximum values of about 40 CFU mL−1 on 7/21/11 and 7/28/11 when the weather was the hottest (Fig. 4). As the maximum temperatures deceased, so did fecal coliform concentrations. E. coli concentrations did not show correlations with temperature. E. coli concentrations of 16 MPN mL−1 were observed on one of the hottest days (7/28/11), whereas they were at the lowest level between 1.5 and 3 MPN mL−1 on the second hottest day (7/21/11).
No relationship was found between concentrations of total coliforms, fecal coliforms, and E. coli in the creek, with one exception. A linear relationship was observed in experiments on 07/14/11 and 07/28/11 between E. coli and fecal coliforms. E. coli concentrations were in the range between 4 and 16 MPN mL−1. The increase in concentrations of E. coli was about 1 MPN mL−1 per 3 CFU mL−1 fecal coliforms (data not shown).
Statistical analysis
Data were analyzed in an attempt to determine whether microbial concentrations in creek and sprinkler water were different; either concentration in water increased due to detachment or release of microbes from biofilms, or conversely, concentrations in water decreased due to microbes from water attaching or being incorporated into biofilms. Two statistical hypotheses were posed for testing: (1) there will be differences in changes in coliform concentrations between water passing through new pipes or used pipes, and (2) coliform concentrations will change when fresh water passes through the pipes.
To address the first hypothesis, the number of cases when the average concentration in sprinkler water was larger than the average concentration in creek was tabulated. Table 4 shows the percentages of such cases for both new and used pipes and for the three microbial groups, and probability that the pipe usage had an influence on the microbial concentration. There was a high probability (P > 0.9) that pipe usage influenced concentration changes for total coliforms and E. coli; a lower probability was found for fecal coliforms.
To address the second hypotheses, we computed probabilities of average concentrations in creek and in sprinkler water being different for each sampling. The most decisive results were obtained for fecal coliforms (Fig. 6). In all cases, the probability of average fecal coliform concentration in creek and sprinkler water being different was larger than 0.5. That is, concentrations of fecal coliforms were altered with high probability (>0.5) while water was moving through pipes. Interestingly, the percentage of cases with the highest probability of average concentration being altered was larger with the new pipe (rightmost bars in Fig. 6). Results for total coliform concentrations resembled the results for fecal coliforms (Fig. 7), although the probability of average total coliform concentration in creek and sprinkler water being different was larger than 0.5 not in all cases as for fecal coliforms, but only in 70 % of cases. That is, in majority of cases, concentrations of fecal coliforms were altered with high probability (>0.5) while water was moving through pipes. On average, the probability of water to be altered was slightly higher in new pipes than in used pipes (compare gray and black bars in Figs. 6, 7).
Figure 8 shows the results for E. coli that appeared to be quite different from the results for fecal and total coliforms. In the majority of cases, the probability of average E. coli concentrations being different in creek and in sprinkler water is low, less than 0.5. Only in 30 % of cases, the probability of average E. coli concentrations being different in creek and in sprinkler water was larger than 0.5. No systematic differences between used and new pipes can be observed.
Discussion
These data document the potential for coliform bacteria to attach to or become incorporated into biofilms on the surface of irrigation pipes. The relevance of this observation is that the concentration and species prevalence of microbes entering irrigation systems may not be identical to what is leaving and, consequently, being applied to produce. The evidence for E. coli attachment or incorporation into biofilms was less pronounced, but the potential clearly exists. In a prior experiment carried out at the same site a year earlier (Pachepsky et al. 2012), E. coli was detected in biofilms in large numbers (in old pipe); average E. coli concentrations in biofilms ranged from 6.4E + 05 MPN m−2 on 25 August to 1.E + 04 MPN m−2 on 15 September. Since E. coli concentrations in the creek were similar for both years, it is unclear why results varied. However, it is well established that E. coli strains are highly variable in terms of their ability to form biofilms (Foppen et al. 2009). There was no evidence that any of the E. coli strains were pathogenic; however, it is reasonable that these strains would behave similarly. These findings are consistent with previous literature documenting biofilm formation in water distribution systems. Although colonization by diverse microorganisms is well established, the evidence for E. coli attachment and colonization is inconsistent. Depending on the strain, 1–50 % of injected E. coli sorbed within a few hours to the indigenous bacterial biofilm in a pilot drinking water system (Fass et al. 1996). Similarly, biofilms presented a hospitable environment for E. coli in a model distribution system in the work of Williams and Braun-Howland (2003). On the other hand, there are reports from laboratory studies in which the development of biofilm on used pipe surfaces was not accompanied by E. coli colonization (e.g., Silhan et al. 2006).
Although the concentrations of coliforms and E. coli were highly variable, a trend was still observed in the relative concentrations of microbes in used versus new pipes. Specifically, concentrations in new pipes tended to be higher than in old pipes. This is counterintuitive in that rough surfaces (found in aged pipes) have been reported to promote attachment and biofilm formation. For example, it is well established that pipe corrosion affects disinfectant efficiency (LeChevallier et al. 1993). However, in this instance, the deterioration of aluminum pipes, resulting in the leaching of aluminum into the water solution, may have had a biocidal effect. There is literature documenting the biocidal affects of aluminum on bacteria in general (Pina and Cervantes 1996) and E. coli specifically (Guida et al. 1991). This is supported by the observation that the concentrations of coliforms in the standing water (1 week after an irrigation event) in new pipes increased (growth) while populations in old pipes decreased (die-off). Since the water and temperatures were essentially identical, the only remaining variable is pipe age/deterioration. Data for E. coli are less clear; however, all concentrations were very low such that any observed differences could be attributable to sampling error.
High daily maximum temperatures observed in mid-summer (up to 54°; Fig. 2) did not appear to adversely impact the bacterial survival. Residual total coliform concentrations were relatively high (Table 2) throughout the season, at least for the new pipe. It is unclear whether this was due to the initial attachment of thermotolerant strains or the subsequent acclimations of strains after attachment. Formation of biofilms at temperatures in the range of temperatures shown in Fig. 2 has been described in domestic hot water systems (Bagh et al. 2004). Escherichia coli survival at high temperatures has been documented in direct evolution experiments. Riehle et al. (2003) demonstrated that E. coli acclimation to long-term temperature stress leads to significant changes at the molecular level in heat-inducible gene expression and at the organism level in inducible thermotolerance and fitness. In the latter work, following acclimation at 41.5 °C, two E. coli lines exhibited significantly improved survival at 50 °C. It was shown in cyclic challenge-resuscitation experiments that E. coli tolerance to temperatures up to 60 °C could be acquired relatively rapidly (Vanlint et al. 2011).
Based on a large number of paired creek and sprinkler water samples, there is a high probability that differences in total and fecal coliform concentration were not random. This was relatively less pronounced for E. coli; although the relatively low concentration of E. coli in biofilms makes comparisons difficult. This is consistent with our prior study where the analysis of variance showed that the probability of the source (sprinkler vs. creek) being an influential factor was 0.86 for the 25 August and 9 September irrigation events and 0.63 for the 1 September irrigation event (Pachepsky et al. 2012). These results indicate that water-borne coliforms and E. coli can either adhere to or be released from the inner surfaces of pipes (e.g., biofilms). Furthermore, both processes probably occur simultaneously in different parts of the pipe during an irrigation event.
Cell death as a mechanism for decreased bacterial numbers coming from sprinklers cannot be excluded. Die-off could be caused by mechanical damage of cells in water flow or by the joint action of malnutrition, predator activity, and elevated temperature. However, since the exposure time for bacteria in pipes was relatively short, it is unlikely that biological and thermal factors had enough time to cause substantial damage. These factors may cause die-off of bacteria at 20 °C in surface waters in the dark, but not faster than one order of magnitude per day (Pachepsky et al. 2006). Similarly, elevated pressure is known to be able to cause E. coli inactivation (Pagan and Mackey 2000). However, pressure in our system was much less than the levels of hundreds MPa that have been shown to cause a significant E. coli die-off (Santillana Farakos and Zwietering 2011). Having said that, we recognize that some elements of irrigation equipment may present unfavorable conditions for bacteria, and die-off within the system remains a possibility, which presents an avenue to explore.
Variability in bacteria concentrations documented in this work is undoubtedly affected by the accuracy of bacterial identification methods. Methods used in this work are known to have an estimated lower detection limit of 1 organism per 100 mL (I.S.H.A 2010) for Colilert and 1 organism per 10 mL for MacConkey agar (Neogen 2011). The accuracy of the Colilert method is deemed to be sufficient in analysis of drinking water (US EPA 2005). The McConkey agar has been used for the same purpose when fecal coliforms were the indicator organisms of choice to evaluate drinking and bathing waters. Note that for all data sets, none of the observations were outside the detection range. One factor affecting the variability in results, using both Colilert and McConkey agar, is the genetic diversity of target bacterial populations since the methods are applied to broad groups of organisms (fecal coliforms, total coliforms, and E. coli), while individual organisms within these groups may exhibit different physiological responses. The accuracy-related variability of bacteria testing results makes the differences between concentrations less well expressed, and essentially decreases the probabilities of concentrations to be different before and after water contact with pipe inner surfaces (Table 4).
Results of this work show that the effect of pipe biofilms on the microbiological quality of irrigation water is a stochastic process with a substantial random component. It is well established that biofilm formation is highly patchy and unpredictable. Camper et al. (1996) noted that locating microbes in drinking water systems plagued with coliform regrowth problems is extremely difficult, presumably due to nonuniform distribution. LeChevallier et al. (1996) observed that that the occurrence of coliform bacteria within a distribution system is dependent upon a complex interaction of chemical, physical, operational, and engineering parameters. This is in agreement with general view on possible modeling of the biofilm action in pipes as a process with both random and deterministic components (Klapper and Dockery 2010).
The fact that biofilms in irrigation equipment may act as a source or sink of microbes poses substantial research questions. Reliable methods of biofilm detection and analysis for irrigation equipment have to be introduced. It is highly probable that the composition of irrigation water may affect the formation of biofilms and their retention ability with respect to water-borne pathogens. It will be important to find out whether and which pathogenic microorganisms can be retained and released in this equipment. Toward this end, differences need to be investigated between materials used in irrigation pipes, valves, nozzles, and other irrigation system components where biofilms can be created. Since the retention and release of microbes appears to be a stochastic process, the probabilities of pathogen retention have to be quantified to include the biofilm factor into risk assessment procedures that have been proposed for microbial quality of irrigation waters (Stine et al. 2005; Mara et al. 2007; Gerba 2009). Sampling of the irrigation waters to assess their microbial quality needs to be done in the field rather than at the intake points. Finally, the feasibility of implementing methods of microbial inactivation to be applied to pipe-based irrigation systems needs to be investigated.
References
Bagh LK, Albrechtsen HJ, Arvin E, Ovesen K (2004) Distribution of bacteria in a domestic hot water system in a Danish apartment building. Water Res 38:225–235
Berry D, Xi C, Raskin L (2006) Microbial ecology of drinking water distribution systems. Curr Opin Biotechnol 17:297–302
Brandl MT (2006) Fitness of human enteric pathogens on plants and implications for food. Annual Revue Phytopathol 44:367–392
Brown D, Rothery P (1993) Models in biology: mathematics, statistics and computing. Wiley and Sons, New York
Camper AK, Jones WL, Hayes JT (1996) Effect of growth conditions and substratum composition on the persistence of coliforms in mixed-population biofilms. Appl Environ Microbiol 62:4014–4018
Chang YC, Le Puila M, Biggerstaff J, Randall AA, Schulte A, Taylor JS (2003) Direct estimation of biofilm density on different pipe material coupons using a specific DNA-probe. Mol Cell Probes 17:237–243
DehghaniSanij H, Yamamoto T, Rasiah V, Utsunomiya J, Inoue M (2004) Impact of biological clogging agents on filter and emitter discharge characteristics of microirrigation systems. Irrigation Drain 53:363–373
Fass S, Dincher ML, Reasoner DJ, Gatel D, Block J-C (1996) Fate of Escherichia coli experimentally injected in a drinking water distribution pilot system. Water Res 30:2215–2221
Foppen JW, Lutterodt G, Röling WFM, Uhlenbrook S (2009) Towards understanding inter-strain attachment variations of Escherichia coli during transport in saturated quartz sand. Water Res 44:1202–1212
Gerba CP (2009) The role of water and water testing in produce safety. In: Fan X, Niemira BA, Doona CJ, Feeherty FE, Gravani RB (eds) Microbial safety of fresh produce. Wiley and Sons, New York, pp 129–142
Guida L, Ssidi Z, Hughes MN, Poole RK (1991) Aluminum toxicity and binding to Escherichia coli. Arch Microbiol 156:507–512
Hallam NB, West JR, Forster CF, Simms J (2001) The potential for biofilm growth in water distribution systems. Water Res 35:4063–4071
I.S.H.A (2010) Validation study of the Colilert®-18/Quanti-Tray® method for the enumeration of Escherichia coli and coliforms in water for human consumption. Summary Report. Institute Scientifiqued’Hygiene & d’Analyse, Massy, France. Available as http://www.idexx.com/pubwebresources/pdf/en_us/water/colilert-18-afnor-report.pdf
Kilb B, Lange B, Schaule G, Flemming HC, Wingender J (2003) Contamination of drinking water by coliforms from biofilms grown on rubber-coated valves. Int J Hyg Environ Health 206:563–573
Klapper I, Dockery J (2010) Mathematical description of microbial biofilms. SIAM Rev 52:221–265
LeChevallier MW, Lowry CD, Lee RG, Gibbon DL (1993) Examining the relationship between iron corrosion and the disinfection of biofilm bacteria. J Am Water Works Assoc 85:111–123
LeChevallier MW, Welch NJ, Smith DR (1996) Full-scale studies of factors related to coliform regrowth in drinking water. Appl Environ Microbiol 62:2201–2211
Lehtola MJ, Nissinen TK, Miettinen IT, Martikainen PJ, Vartiainen T (2004a) Removal of soft deposits from the distribution system improves the drinking water quality. Water Res 38:601–610
Lehtola MJ, Miettinen IT, Keinanen MM, Kekki TK, Laine O, Hirvonen A, Vartiainen T, Martikainen PJ (2004b) Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res 38:3769–3779
Li Y, Zhou B, Liu Y, Jiang Y, Pei Y, Shi Z (2012) Preliminary surface topographical characteristics of biofilms attached on drip irrigation emitters using reclaimed water. Irrigation science. Published online. doi:10.1007/s00271-012-0329-1
Mara DD, Sleigh PA, Blumenthal UJ, Carr RM (2007) Health risks in wastewater irrigation: comparing estimates from quantitative microbial risk analyses and epidemiological studies. J Water Health 5:39–50
Morton SC, Zhang Y, Edwards MA (2005) Implications of nutrient release from iron metal for microbial regrowth in water distribution systems. Water Res 39:2883–2892
Neogen (2011) MacConkey agar. Available at http://www.neogen.com/Acumedia/pdf/ProdInfo/7102_PI.pdf
Pachepsky Y, Sadeghi AM, Bradford SA, Shelton DR, Guber AK, Dao T (2006) Transport and fate of manure-borne pathogens: modeling perspective. Agric Water Manag 86:81–92
Pachepsky Y, Shelton D, McLain J, Patel J, Mandrell R (2011) Irrigation waters as a source of pathogenic microorganisms in produce: a review. Adv Agron 113:71–136
Pachepsky Y, Morrow J, Guber A, Shelton D, Rowland R, Davies G (2012) Effect of biofilm in irrigation pipes on microbial quality of irrigation water. Lett Appl Microbiol. doi:10.1111/j.1472-765X.2011.03192.x
Pagan R, Mackey B (2000) Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential- and stationary-phase cells and variation among strains. Appl Environ Microbiol 66: 2829–2834
Pina RG, Cervantes C (1996) Microbial interactions with aluminum. Biometals 9:311–316
Riehle MM, Bennett AF, Lenski RE, Long AD (2003) Evolutionary changes in heat-inducible gene expression in lines of Escherichia coli adapted to high temperature. Physiol Genomics 14:47–58
Sadovski AY, Fattal B, Goldberg D, Katzenelson E, Shuval HI (1978) High levels of microbial contamination of vegetables irrigated with wastewater by the drip method. Appl Environ Microbiol 36:824–830
Santillana Farakos SM, Zwietering MH (2011) Data analysis of the inactivation of foodborne microorganisms under high hydrostatic pressure to establish global kinetic parameters and influencing factors. J Food Prot 74:2097–2106
Silhan J, Corfitzen CB, Albrechtsen HJ (2006) Effect of temperature and pipe material on biofilm formation and survival of Escherichia coli in used drinking water pipes: a laboratory-based study. Water Sci Technol 54:49–56
Stine SW, Song I, Choi CY, Gerba CP (2005) Application of microbial risk assessment to the development of standards for enteric pathogens in water used to irrigate fresh produce. J Food Prot 68:913–918
US EPA (2002a) Health risks from microbial growth and biofilms in drinking water distribution systems. http://www.epa.gov/ogwdw/disinfection/tcr/pdfs/whitepaper_tcr_biofilms.pdf. Accessed on 2/4/2012
US EPA (2002b) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th ed. EPA 821-R-02-012, Section 7.2.3.1, p 32. US EPA, Office of Water, Washington, DC
US EPA (2005) Manual for the certification of laboratories analyzing drinking water.criteria and procedures. Quality Assurance. Fifth Edition. EPA 815-R-05-004. US Environmental Protection Agency. Office of Water. Office of Ground Water and Drinking Water. Technical Support Center. Cincinnati, Ohio 45268
US CDC (2012) Foodborne Outbreak Online Database (FOOD). http://wwwn.cdc.gov/foodborneoutbreaks/Default.aspx. Accessed on 2/14/2012
USGS (2012) USGS Water-Quality Data for Maryland. Available at the web at http://waterdata.usgs.gov/md/nwis/qw. Accessed on 03/22/2012
Vanlint D, Mitchell R, Bailey E, Meersman F, McMillan PF, Michiels CW, Aertsen A (2011) Rapid acquisition of gigapascal-high-pressure resistance by Escherichia coli. mBio 2(1):e00130-10. doi:10.1128/mBio.00130-10
Williams MW, Braun-Howland EB (2003) Growth of Escherichia coli in model distribution system biofilms exposed to hypochlorous acid or monochloramine. Appl Environ Microbiol 69:5463–5471
Yu J, Kim D, Lee T (2010) Microbial diversity in biofilms on water distribution pipes of different materials. Water Sci Technol 61:163–171
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Stone.
Rights and permissions
About this article
Cite this article
Shelton, D.R., Kiefer, L.A., Pachepsky, Y.A. et al. Coliform retention and release in biofilms formed on new and weathered irrigation pipes. Irrig Sci 31, 971–981 (2013). https://doi.org/10.1007/s00271-012-0373-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00271-012-0373-x