Abstract
Purpose
This study aims to evaluate the intra-procedural use of a novel ablation confirmation (AC) method, consisting of biomechanical deformable image registration incorporating AI-based auto-segmentation, and its impact on tumor coverage by quantitative three-dimensional minimal ablative margin (MAM) CT-generated assessment.
Materials and methods
This single-center, randomized, phase II, intent-to-treat trial is enrolling 100 subjects with primary and secondary liver tumors (≤ 3 tumors, 1–5 cm in diameter) undergoing microwave or radiofrequency ablation with a goal of achieving ≥ 5 mm MAM. For the experimental arm, the proposed novel AC method is utilized for ablation applicator(s) placement verification and MAM assessment. For the control arm, the same variables are assessed by visual inspection and anatomical landmarks-based quantitative measurements aided by co-registration of pre- and post-ablation contrast-enhanced CT images. The primary objective is to evaluate the impact of the proposed AC method on the MAM. Secondary objectives are 2-year LTP-free survival, complication rates, quality of life, liver function, other oncological outcomes, and impact of AC method on procedure workflow.
Discussion
The COVER-ALL trial will provide information on the role of a biomechanical deformable image registration-based ablation confirmation method incorporating AI-based auto-segmentation for improving MAM, which might translate in improvements of liver ablation efficacy.
Conclusion
The COVER-ALL trial aims to provide information on the role of a novel intra-procedural AC method for improving MAM, which might translate in improvements of liver ablation efficacy.
Trial registration
ClinicalTrials.gov identifier: NCT04083378.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver cancer is a leading cause of cancer deaths globally [1]. Local therapies that are safe, cost-effective, and repeatable are urgently needed. Percutaneous thermal ablation has become a widely utilized local therapy for patients with both primary and secondary liver cancers not eligible for surgical resection, with recent series demonstrating encouraging overall survival rates in par with surgical resection in selected patients [2,3,4,5,6,7,8]. Nevertheless, its associated higher rates of local recurrence and disease-free survival when compared to surgical resection still remains as a major limitation for its widespread acceptance [4]; [9,10,11,12,13,14,15].
Complete tumor coverage with minimal ablative margin (MAM) with at least ≥ 5 mm is considered one of the major factors associated with improved local tumor control following thermal ablation [16,17,18,19,20,21]. Moreover, it is the only prognostic factor that can be modified during delivery of ablation therapy. Aside from proper patient selection, achieving sufficient MAM requires proper tumor mapping, targeting, and margins evaluation. Unfortunately, several intrinsic factors related to ablation therapy poses challenges to achieve such required steps. Namely, changes in liver shape, position, and volume due to patient’s breathing and ablation-related tissue dehydration, image quality degradation due to probe placement, hydrodissection, and limitations on contrast-media dose exposure are known limitations for ablation therapy accuracy [10]; [22,23,24].
The retrospective evaluation of ablation confirmation (AC) software packages on patients previously submitted to percutaneous thermal ablation has been reported by several investigators, demonstrating a correlation between local tumor progression (LTP) and MAM thresholds estimated by such AC methods [16,17,18,19,20]. Nevertheless, the prospective use of ablation confirmation (AC) methods intra-procedurally and its impact on ablation outcomes, along with its potential consequent clinical benefits, remains unclear, currently limiting the standardization and validation of such methods. We hypothesize that MAM following percutaneous thermal ablation of liver cancers will be significantly improved with the use of a novel AC method consisting of a deformable image registration (DIR) method incorporating ablation-specific artificial intelligence (AI)-based auto-segmentation. This enables accurate mapping of the target tumor, verifying proper ablation applicator(s) tumor targeting, and assessing MAM intra-procedurally, while taking in account the intrinsic factors that currently limits such required steps. Therefore, the proposed AC method can potentially improve local oncological outcomes.
Materials and Methods
Trial Design and Study Setting
The COVER-ALL trial is a prospective, randomized, two-arm, intent-to-treat phase II study, being conducted at The University of Texas MD Anderson Cancer Center. The trial started recruitment in January 2020. This study is funded by the National Institutes of Health.
Study Population
Patients referred to percutaneous liver ablation at the Interventional Radiology Department will be screened for trial eligibility and enrolment based on the review of the electronic medical chart. Subjects over 18 years with confirmed primary and secondary liver cancers planned to undergo percutaneous thermal ablation as per standard of care are eligible. Subjects can only be enrolled in the study once. Full inclusion and exclusion criteria are provided in Table 1.
Interventions
The ablation procedure, peri-procedural care, and follow-up will be performed in accordance with our standard of care institutional practice. All ablations are performed under CT-guidance under general anesthesia support. All CT images are acquired during apnea. Target tumors are treated with the intent to obtain complete tumor ablation with ≥ 5 mm MAM. Only radiofrequency ablation (Cool-tip, Medtronic Inc, Dublin, Ireland) and microwave ablation (Neuwave, Ethicon inc, Raritan, NJ, USA) are allowed in this study. Given our current practice, we expect that over 95% of enrolled patients will be treated with microwave ablation. Hydrodissection or other adjunctive techniques to prevent thermal damage to adjacent critical structures are allowed during any steps of the ablation procedure.
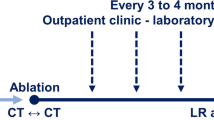
A schematic review of the study workflow is depicted on Fig. 1. A dual-phase pre-ablation contrast-enhanced CT will acquired to identify the target tumor and confirm trial eligibility using a standardized CT imaging protocol (Appendix 1). After percutaneous placement of ablation applicator(s), a native CT will be acquired to verify the position of ablation applicator(s) at the target tumor by visual inspection. Then, both pre-ablation native and contrast-enhanced CT images with ablation applicator(s) will be transferred to RayStation treatment planning system (RaySearch Laboratories, Stockholm, Sweden) for AI-based auto-segmentation and biomechanical DIR for target tumor mapping on native CT image (Appendix 2). The results will not be disclosed to the interventional radiologist before randomization. The subject will not be enrolled if tumor cannot be well visualized or segmented. After ablation applicator placement is deemed appropriate by the interventional radiologist, subjects will be randomized. For the experimental arm, the spatial correlation between the target tumor and ablation applicator(s) will be disclosed to the interventional radiologist on 2D and 3D images. Reposition of ablation applicator(s) and ablation will be performed accordingly to interventional radiologist’s discretion. For the control arm, the information generated by the AI-based auto-segmentation and biomechanical DIR will not be disclosed to the interventional radiologist.
After ablation delivery, a post-ablation dual-phase contrast-enhanced CT will be performed to confirm tumor coverage by ablation zone and MAM quantification. Pre- and post-ablation contrast-enhanced CT images will be transferred to RayStation for biomechanical DIR and AI-based auto-segmentation. Then, the target tumor will be mapped onto post-ablation contrast-enhanced CT image and the MAM will be automatically quantified (Fig. 2). For the experimental arm, MAM quantification generated by the AC method will be disclosed to the interventional radiologist, along with its spatial localization on 2D and 3D images. For the control arm, the results of MAM quantification will not be disclosed to the interventional radiologist, who will determine the MAM as per our current standard of practice, consisting of anatomical landmarks-based quantitative measurements aided by co-registration of pre- and post-ablation contrast-enhanced CT images [16]. Re-ablation is permitted in both arms. Final MAM quantification will be performed utilizing the final post-ablation contrast-enhanced CT.
Artificial intelligence-based auto-segmentation, biomechanical deformable image registration, spatial correlation between the target tumor and ablation applicator(s), and minimal ablative margin quantification in a 57-year-old man with colorectal liver metastasis treated with microwave ablation. A) pre-ablation contrast-enhanced CT, auto-segmentation of liver (light blue) and target tumor (green). B) After ablation applicators placement, a native CT was acquired to verify the position of ablation applicators (arrows). Then, biomechanical deformable image registration was performed with both pre-ablation contrast-enhanced CT and native CT and the target tumor (green) was mapped on native CT. C) 3D volume rendering image of native CT for spatial correlation between the target tumor (green) and ablation applicators (dark blue). D) A final contrast-enhanced CT was acquired to verify ablation zone (orange). Then, biomechanical deformable image registration was performed with both pre- and post-ablation contrast-enhanced CT and the target tumor (green) was mapped on post-ablation contrast-enhanced CT. The 3D minimal ablative margin was computed, which was 5.5 mm, located on a plane between the sagittal and coronal planes (not shown)
Randomization methodology
Subjects will be randomly assigned 1:1 to two treatment arms using the Pocock-Simon dynamic allocation method [25] with a minimization probability parameter of 0.90 to balance the baseline covariates: tumor histology (colorectal liver metastases or other histology), RAS mutation status (for colorectal liver metastasis only, mutated, wild-type, or undetermined), tumor size (< 2 cm, 2 to 3 cm, or > 3 to ≤ 5 cm), subcapsular location (defined as tumor within 1 cm from the liver capsule, yes or no), and presence of multiple tumors (yes or no).
Follow-up
Subjects will be followed up to 24 months after intervention. Subjects will undergo chest and abdominal contrast-enhanced CT or contrast-enhanced liver magnetic resonance imaging 4 to 12 weeks after the ablation procedure. Then, chest and abdominal contrast-enhanced CT, contrast-enhanced liver magnetic resonance imaging, or fluorine 18 fluorodeoxyglucose positron emission tomography will be acquired 3–4 months thereafter until death per institutional practice. Follow-up images will be reviewed by two experienced abdominal radiologists to determine residual unablated tumor, LTP, intrahepatic tumor progression other than target tumor, and extrahepatic disease progression. The abdominal radiologists will be blinded to the allocation of subjects (experimental vs control arms). The interpreting radiologists will be blinded to the results of MAM and disagreements in imaging interpretation will be resolved by consensus. All the ablation outcomes will be assessed according to image-guided tumor ablation standardized terminology and reporting criteria [26]; [27].
Objectives and outcomes
Clinical trial schema is depicted on Fig. 3. The primary objective is to evaluate if the intra-procedural feedback of the proposed AC method (biomechanical DIR incorporating ablation-specific AI-based auto-segmentation) will increase the MAM on a three-dimensional computed tomography-generated analysis. Secondary objectives are assessing whether applying the proposed AC method improves 2-year LTP-free survival (LTPFS) rates and other oncological outcomes (i.e., intra-hepatic and overall progression-free survivals and overall survival), and to evaluate the impact of its use on procedure workflow, complication rates, quality of life, and liver function.
Sample size
In order to avoid within patient correlation for subjects with multiple tumors, only the largest tumor per subject will be evaluated on this trial. A retrospective analysis at our institution using the proposed AC method demonstrated a mean MAM of 2 mm (standard deviation of 2 mm) without proposed AC method guiding intra-procedurally [28]. Assuming the pooled standard deviation of MAM is 2 mm, a sample size of 50 evaluable subjects in each arm will have 80% power to detect a difference of 1.132 mm using an independent 2-sample t-test with a two-sided 0.05 level of significance. In addition, we expect approximately 20 subjects to be screen failures and 20% subjects to be dropout after randomization due to tumor progression, inability to clearly depict target tumors on contrast-enhanced CT, technical limitations, and complications that will preclude further ablation. To account these unevaluable subjects, we will screen a total of 140 subjects and enroll 120 subjects to ensure 100 evaluable unique subjects. Assuming an accrual rate of 35 subjects per year, the study accrual duration will be around 3 years, with the total study duration around 5 years to account for a follow-up period of at least 2 years.
Interim analysis
An interim look for superiority with the use of the proposed AC method will be performed once half the evaluable subjects (n = 50) have been enrolled. A Lan-Demets α-spending function using an Obrien-Flemming boundary will be used for superiority stopping boundaries [29]. We will stop enrolment at the control arm at our interim look if the differences on the MAM between the two arms disclose a p-value less than 0.003. In that case, the next 50 subjects will be enrolled on the experimental arm only to allow further development of the proposed method on clinical practice and allow more interventional radiologists at our institution to participate in this trial. East v6.5 (Cytel, Cambridge, MA, USA) was used for sample size calculation.
Statistical methods
For the primary objective, the average MAM will be compared between two arms using a 2-sample t-test (or Wilcoxon rank-sum test). The means and corresponding 95% confidence intervals will be reported for both arms. As secondary objectives, the Kaplan–Meier method will be used to estimate LTPFS and 95% confidence intervals for the quantiles of the survival function based on the method of Brookmeyer and Crowley [30] will be calculate. Time point probabilities (e.g., 2-year LTPFS) and the associated log–log transformed pointwise 95% confidence will also be reported. A multivariate Cox-proportional hazards model will be fitted to the data with MAM as a continuous variable to assess the significance of MAM on LTPFS while simultaneously adjusting for other known risk factors. LTPFS will be measured from date of ablation to earliest date of progression at the ablated tumor or death. Those progression free and alive will be censored at their date of last clinic visit. Similar analysis will be used for 2-years intra-hepatic progression-free survival and overall survival. Standard summary statistics will be computed for complication rates, quality of life, and liver function and compared between both arms. Statistical significance will be defined as p < 0.05.
Discussion
The increased use of thermal ablation as an alternative to surgical resection has been predicated by its minimally invasive nature, rational use of rising healthcare costs, lower complication rates, and faster recovery [9]; [11]; [13]; [31]. Although studies have shown similar oncological outcomes between thermal ablation and surgery for small primary liver cancers, historically worse rates of local recurrence following thermal ablation when compared to surgery have hindered its application as a first local curative-intent modality for patients with primary and secondary liver cancers.
Several investigators retrospectively evaluated the impact of MAM on local tumor control rates, demonstrating an association of larger MAM with improved local tumor control. Currently, providing potential differences in respect tumor histology and subtypes, the optimal MAM is recommended to be at least ≥ 5 mm [16,17,18,19,20]. Nevertheless, such recommendations are based on retrospective data utilizing cross-sectional imaging that were not obtained intra-procedurally. Therefore, its extrapolation as an immediate surrogate for MAM is not possible given that immediate ablation-related changes depicted on imaging were not factored in. Moreover, the use of cross-sectional images acquired several days/weeks after the ablation to account for MAM also adds significant challenges for accounting tissue contraction into MAM estimation. Finally, manual segmentation and registration of tumor and ablation zones invariably adds operator bias on MAM analysis. We believe that the use of AI-based methods for tumor and ablation zone segmentations as proposed in this present study is poised to reduce operator input and consequent associated biases on MAM quantification.
Currently, the prospective use of AC methods for intra-procedural decision-making on an intent-to-treat approach and its consequent potential translation into clinical benefit remains elusive. It is expected that the COVER-ALL trial will allow to specifically evaluate the impact of the proposed AC method as an intra-procedural tool for decision-making and MAM quantification. It is also expected that this study will allow us to understand the impact of the use of this AC method on procedure workflow. We speculate that the use of the proposed AC method based on a DIR incorporating ablation-specific AI-based auto-segmentation for ablation applicator placement verification and MAM quantification will allow optimal coverage of the target tumor and surrounding tissue at risk of progression, while reducing the ablation of non-target tissue (i.e., surrounding non-tumor liver parenchyma tissue).
Our study design has limitations. Firstly, this is a histology-agnostic study, which might limit the correlation between the MAM quantification and LTP outcomes, which is a secondary objective of the study. This limitation arises from the single-center nature of this study, which would make significantly challenging to accrue the required number of patients with a single tumor histology. Moreover, this study has been designed to translate a novel AC methodology consisting of DIR and artificial intelligence-based tumor and ablation zones segmentation into clinical practice. Therefore, a larger patient population with a wider variety of tumor types would better reflect the current unmet needs in clinical practice. Multi-institutional studies investigating the use of AC methods focused on specific tumor histologies such as the Prometheus (hepatocellular carcinoma) and ACCLAIM (colorectal liver metastasis) trials are better suited to correlate ablation margins with local tumor outcomes [32]; [33]. Secondly, given the current constrains in performing AI-based segmentation on tumors < 1 cm, sub-centimeters tumors are excluded from the trial, adding a selection bias to trial design. Third, acquisition of more than one post-ablation contrast-enhanced CT might be required if intra-procedural MAM ≥ 5 mm is not achieved in first attempt, which may increase the risk of contrast-associated acute kidney injury. Thus, we only include subjects with preserved renal function in the trial. Finally, due to the unblinded nature of this study, it is conceivable that operator bias might occur in respect performing more extensive ablations and more careful planning in one of the study’s arms. In order to gain further insight on this potential bias, we will perform volumetric analysis on the amount of non-tumorous liver parenchyma ablated tissue between the two arms.
In conclusion, the COVER-ALL trial aims to provide information on the role of a novel intra-procedural ablation confirmation method for improving MAM, which might translate in improvements of liver ablation efficacy. Trial registration: ClinicalTrials.gov identifier: NCT04083378.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–68.
Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–A 10-year experience at a single center. Radiology. 2016;278(2):601–11.
Shi J, Sun Q, Wang Y, et al. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol. 2014;29(7):1500–7.
Shiina S, Tateishi R, Arano T, et al. (2012) Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol, 107(4):569–577; quiz 578
Peng ZW, Zhang YJ, Chen MS, Lin XJ, Liang HH, Shi M. Radiofrequency ablation as first-line treatment for small solitary hepatocellular carcinoma: long-term results. Eur J Surg Oncol. 2010;36(11):1054–60.
Wang Z, Liu M, Zhang DZ, et al. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5-cm HCC. Hepatology. 2022;76(1):66–77.
Bale R, Widmann G, Schullian P, et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol. 2012;22(4):930–7.
Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K (2013) Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev(12):Cd003046
Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25(12):3438–54.
Abdalla E K, Vauthey J-N, Ellis L M, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Annals of Surgery. 2004;239(6):818–27. https://doi.org/10.1097/01.sla.0000128305.90650.71.
Di Martino M, Rompianesi G, Mora-Guzmán I, Martín-Pérez E, Montalti R, Troisi RI. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur J Surg Oncol. 2020;46(5):772–81.
Otto G, Düber C, Hoppe-Lotichius M, König J, Heise M, Pitton MB. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg. 2010;251(5):796–803.
Nishiwada S, Ko S, Mukogawa T, et al. Comparison between percutaneous radiofrequency ablation and surgical hepatectomy focusing on local disease control rate for colorectal liver metastases. Hepatogastroenterology. 2014;61(130):436–41.
Kron P, Linecker M, Jones RP, Toogood GJ, Clavien PA, Lodge JPA. Ablation or Resection for Colorectal Liver Metastases? A Systematic Review of the Literature. Front Oncol. 2019;9:1052.
Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–75.
Shady W, Petre EN, Do KG, et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J Vasc Interv Radiol. 2018;29(2):268-275.e261.
Shady W, Petre EN, Vakiani E, et al. (2017) Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget, 8(39)
Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28(7):2727–34.
Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188(2):480–8.
Laimer G, Jaschke N, Schullian P, et al. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur Radiol. 2021;31(9):6489–99.
Brace CL, Diaz TA, Hinshaw JL, Lee FT Jr. Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol. 2010;21(8):1280–6.
Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–71.
van Duijnhoven FH, Jansen MC, Junggeburt JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13(5):651–8.
Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15.
Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–60.
Puijk RS, Ahmed M, Adam A, et al. Consensus Guidelines for the Definition of Time-to-Event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative. Radiology. 2021;301(3):533–40.
Anderson BM, Lin YM, Lin EY, et al. A novel use of biomechanical model-based deformable image registration (DIR) for assessing colorectal liver metastases ablation outcomes. Med Phys. 2021;48(10):6226–36.
Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiley & Sons; 2011.
Brookmeyer R, Crowley J. A Confidence Interval for the Median Survival Time. Biometrics. 1982;38(1):29–41.
Mulier S, Ni Y, Jamart J, Michel L, Marchal G, Ruers T. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15(1):144–57.
Oosterveer TTM, van Erp GCM, Hendriks P, et al. Study Protocol PROMETHEUS: Prospective Multicenter Study to Evaluate the Correlation Between Safety Margin and Local Recurrence After Thermal Ablation Using Image Co-registration in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2022;45(5):606–12.
Sofocleous CT Ablation With Confirmation of Colorectal Liver Metastases (ACCLAIM), ClinicalTrials.gov identifier: NCT05265169. Updated March 3, 2022. Accessed July 7, 2022.
Funding
This study is funded by the National Institutes of Health grants: 1R01CA235564.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kristy Brock received funding from RaySearch Laboratories AB through a Co-Development and Collaboration Agreement. Kristy Brock has a licensing agreement with RaySearch Laboratories AB.
Consent for Publication
For this type of study, consent for publication is not required.
Human or Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent will be obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, YM., Paolucci, I., Anderson, B.M. et al. Study Protocol COVER-ALL: Clinical Impact of a Volumetric Image Method for Confirming Tumour Coverage with Ablation on Patients with Malignant Liver Lesions. Cardiovasc Intervent Radiol 45, 1860–1867 (2022). https://doi.org/10.1007/s00270-022-03255-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03255-3