Abstract
Trans-arterial radioembolization is currently performed using 90Y-loaded glass or resin microspheres and also using 166Ho-loaded microspheres. The goal of this review is to present dosimetry and radiobiology concepts, the different dosimetry approaches available (simulation-based dosimetry and post-treatment dosimetry), main confounding factors as main clinical dosimetry results provided during the last decade for both hepatocellular carcinoma (HCC) and metastases of colorectal carcinoma (mCRC). Based on the different number of microspheres or different isotope used, radiobiology of the three devices is different, meaning that tumouricidal doses and maximal tolerated doses are different. Tumouricidal doses described for HCCs were 100–120 grays (Gy) with 90Y resin microspheres and 205 Gy with 90Y glass microspheres. For mCRC, it is 39–60 with 90Y resin microspheres, 139 Gy with 90Y glass microspheres and 90 Gy with 166Ho microspheres. An impact of tumoural doses with overall survival has also been reported. Personalised dosimetry has been developed and is now recommended by several international expert groups. Level-one evidence of the major impact of personalised dosimetry on response and overall survival in HCC is now available, bringing a new standard approach for TARE in clinical practice as well as for trial design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In liver cancer, trans-arterial radioembolization (TARE) currently uses 90Y-loaded microspheres, either glass microspheres (TheraSphere®, Boston Scientific Corporation, USA) or resin microspheres (SIR-Sphere®, Sirtex Medical Limited Australia), and more recently, 166Ho-loaded microsphere (Quiremspheres®, Terumo Europe). The treatment itself (injection of 90Y- or 166Ho-loaded microspheres) is always preceded by a simulation (currently called work-up) including a diagnostic liver angiography with intra-arterial injection, at the treatment position, of 99mTc macro-aggregated albumin (MAA) to perform a liver perfusion scintigraphy (MAA scan) [1]. For 166Ho-loaded microspheres, the simulation can also be performed with a scout dose of 166Ho-loaded microspheres (Ho-scout) [2].
The goal of TARE is to deliver a tumouricidally absorbed dose to tumours, while sparing normal liver tissue and radiobiological rules apply [1]. For a deterministic radio-induced effect, a threshold-absorbed dose is mandatory to achieve to observe an effect, and the higher the absorbed dose is above this threshold, the more severe the effect is, up to the maximal effect achievable (complete pathological necrosis). Dosimetry is thus a key point for treatment planning, as with external beam radiotherapy.
However, treatment planning is usually based either on an activity of 90Y, expressed in giga-becquerels (GBq), to administer related to the body surface area (BSA) with SIR-Sphere® [3] or on an absorbed dose delivered to the liver: 80 to 150 grays (Gy) with TheraSphere® [4] or 60 Gy with Quiremspheres® [5]. But in reality, TARE planning should be based on the tumouricidal tumour dose necessary to reach to induce a tumour response, and on the maximal normal liver-tolerated dose to minimise liver damage.
Two dosimetry approaches are available with TARE. The first is simulation-based dosimetry (using the MAA quantification or Ho-scout quantification) prior to the treatment, allowing potential dosimetry personalisation. The second is direct 90Y or 166Ho quantification after the treatment, which is assumed to be more accurate but does not allow for personalised dosimetry. Many dosimetry studies have been performed, mainly during the last decade, and have contributed to a new refinement of TARE based on personalised dosimetry.
Dosimetry Concept
From a physical point of view, an absorbed dose is an energy, expressed in Joule (J) divided by a mass, expressed in kilogram (Kg). Absorbed dose is expressed in J/Kg or in Gy with 1 Gy = 1 J/Kg.
One difficulty is that the radiobiological effect depends not only on the absorbed dose, but also on the dose rate and the heterogeneity of the dose distribution, meaning that the same absorbed dose will not provide the same tissue damage if the dose rate or the heterogeneity of the dose distribution is different [1]. In this situation, tissue damage is higher if heterogeneity is lower [1].
With external beam radiation therapy (EBRT), the dose distribution is homogeneous, as radiation is provided by an external gantry that is fully physically calibrated. This differs with TARE, as the dose distribution depends on the biodistribution of the radio-labelled device injected, which is heterogeneous by nature [1].
Due to the difference in specific activity between 90Y-loaded microspheres (50 Bq/ sphere for resin and 2500 Bq/sphere for glass, at calibration time) leading to a difference in the heterogeneity of the dose distribution (and due to a different physical half-life between 90Y and 166Ho), the radiobiological properties of each product are different, meaning that tumouricidal doses and maximal tolerated doses are different between each product and must be evaluated separately. This point has been evaluated in the simulation study of Walrand et al. [6], demonstrating that the dose leading to 50% of normal tissue damage was lower using 90Y-loaded resin microspheres (40 Gy for a whole liver irradiation) than using 90Y-loaded glass microspheres (60 Gy). This difference was explained by the higher number of 90Y-loaded resin microspheres injected for the same activity (about 50-fold more resin spheres than glass spheres related on their difference of specific activity), which was responsible for a more homogeneous sphere distribution with resin microspheres and thus a higher radiobiological effect.
The difference in the number of microspheres injected for the same activity can also have a potential impact on the embolic effect, with a higher embolic effect with 90Y-loaded resin microsphere, which might have a potential therapeutic effect, especially in the situation of poorly vascularised lesion such as metastases of colorectal carcinoma [1].
It has also been demonstrated that for the same device, specific activity has an impact on the dose distribution at the microscopic level for a same absorbed dose delivered at a macroscopic level. Indeed, a study conducted on pigs with 90Y-loaded glass microspheres [7] found that for an absorbed dose of 50 Gy delivered to the normal liver, at a microscopic level, the volume of the treated liver receiving an absorbed dose higher than 30 Gy was only 28.7% for a high specific activity of 1532 Bq/microsphere (injection on day 4 after calibration) in comparison with 60.1% for a lower specific activity of 193 Bq/sphere (injection on day 12 after calibration).
These two studies underline the complexity of radiobiology of TARE, which depends not only on the absorbed dose, but also on the way it is delivered.
Dose Calculation, Medical Internal Radiation Dose (MIRD) Approach
Several dosimetric approaches are well-recognised: the simplest and most widely used is the MIRD approach; more complex approaches such as the biological effective dose evaluation (BED), uniform equivalent dose calculation (EUD), Monte Carlo simulation and kernel point evaluation [1, 8] are also used in some studies.
The MIRD approach assumes a homogeneous distribution of the doses. As microspheres are not biodegradable and remain trapped in the vessels after initial administration, the effective half-life is supposed to be the physical half-life of the radioactive isotope (90Y or 166Ho) thus simplifying the MIRD equation.
The energy deposition in a mass of 1 kg is 50 Gy for 1 GBq of 90Y, and 15.87 Gy for 1 GBq of 166Ho [6].
The absorbed dose “D” (Gy) delivered to a structure (also currently called Volume of Interest (VOI) or compartment), of a mass “M” (Kg), containing an activity “A” (GBq) of 90Y is then calculated using the following simplified MIRD formula (which is the same for resin or glass microspheres):
For 166Ho-loaded microspheres, the simplified MIRD formula is:
Usually, the mass of the liver (in Kg) is assumed to be equal to its volume expressed in litres (L) multiplied by 1.03, and the mass of lungs is assumed to be equal to 1 kg.
Doses can be calculated for different VOIs: perfused liver, tumour, normal perfused liver, and lung tissues. Doses can also be extrapolated to the whole liver and the whole normal liver (then taking into account the non-treated liver volume).
In uni-compartment dosimetry, the dose is evaluated only for one VOI, usually for the perfused volume. This is the standard dosimetry approach used for 90Y glass microspheres [4] and 166Ho microspheres [5].
In the multi-compartment dosimetry, doses are evaluated for several VOI, including the tumour and the normal perfused liver.
Tumour control probability (TCP) curves (providing the probability of control for a tumour-absorbed dose) as well as non-tumour complication probability (NTCP) curves (probability of complication for a normal perfused liver dose) can be generated.
Doses can be evaluated for the whole VOI; this is the mean dose evaluation, which is the simplest approach. Doses can also be evaluated for each voxel of the VOI, which is called voxel dosimetry. Voxel dosimetry allows for the generation of dose-volume histogram (DVH), then mixed metrics based on doses and volumes can be generated as the “Dx” which is the minimum dose received by x% of the volume of the related structure (for example, for a tumour, D70 is the minimal dose received by a least 70% of the volume of the tumour), or the “Vy” with is the volume (%) receiving a dose ≥ y Gy (for example, for the normal liver, the V100 is the percentage of the volume of the normal liver receiving at least 100 Gy). These kinds of metrics, currently used with EBRT, are not widely used with TARE and have to be evaluated as they may contribute to new improvements in dosimetry.
Technical Issues and Confounding Factors for Dosimetry Evaluation
Many potential technical issues or confounding factors have to be highlighted in order to have a good understanding of dosimetry, and to be aware of the reasons why several dosimetry studies have brought sometimes poor or contradictory results.
Segmentation Approach
For the segmentation of the volume of interest (and therefore for the volumes evaluation), two approaches are available [1].
The first is diagnostic imaging using computed tomography (CT), magnetic resonance imaging (MRI), or cone beam computed tomography (CBCT). The imaging needs to be co-registered with single photon computed tomography/computed tomography (SPECT/CT) or positron emission tomography PET to evaluate the count number in the VOI. In this situation, only the counts within the anatomically delineated VOIs are taken into consideration for the dose calculation of this VOI. The advantage of this approach is that it achieves the most accurate and reproducible volume definition. However, in case of co-registration error, significant underestimation of the absorbed dose of this VOI can be observed due to an underestimation of the counts in the VOI.
The second approach available is based on a full SPECT/CT (or PET/CT) segmentation, semi-automated and threshold-based, previously validated by a phantom study where the mean error in the volume measurement was lower than 7%, with good reproducibility [9]. In this situation, the segmentation provides both the volume and the counts included in this volume (no co-registration required). However, in some complex cases, the thresholding may be difficult to perform, with potential error in volume assessment.
To overcome segmentation difficulties, a mixed approach can be used: first, an evaluation of volume based on anatomical tools, and a SPECT/CT or PET/CT segmentation with an optimisation of the thresholding to generate volumes closer to anatomical volume (no co-registration warranted, minimisation of volume error potentially generated by SPECT/CT or PET/CT segmentation alone).
The impact of this segmentation approach has been evaluated in a retrospective multicentric study on hepatocellular carcinoma (HCC) patients (TARGET study, Boston scientific); full results are pending.
Blood Flow Preservation and Reproducibility
Blood flow preservation is a major point being discussed more and more when evaluating dosimetry, especially for simulation-based dosimetry, regardless of the microsphere surrogate used (MAA or Ho-scout). Indeed, simulation-based dosimetry cannot be limited to an accurate quantification of the surrogate itself, but is a global approach including angiographic considerations as blood flow and reproducibility between the work-up and the treatment. Several technical issues may impair the blood flow and reproducibility, such as spasm occurrence [1, 10, 11], proximity of arterial bifurcation [12], speed of surrogate injection [1], and catheter repositioning [13, 14]. Impairment of the blood flow must be researched in each procedure and can be suspected in cases of discrepancy between tumour targeting expected for hypervascularised lesions identified on anatomical imaging (CT/ MRI) and tumour targeting based on CBCT or MAA SPECT/CT.
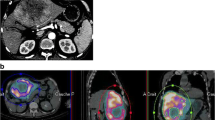
A strong discrepancy between CT and MRI vascularity and CBCT or MAA tumoural targeting means that the simulation is not accurate due to blood flow impairment and that the simulation-based dosimetry will not be accurate (Fig. 1). In this situation, a new simulation has to be considered.
Evaluation of the concordance between MAA targeting and tumour vascularity. A Large hypervascularised HCC on CT scan B First simulation with a MAA SPECT/CT showing a clear discordance between MAA targeting and tumour vascularity with about 40% of the tumour not targeted with MAA, suggesting the occurrence of a blood flow impairment and indicating that in this situation MAA-based dosimetry will not be accurate. For this case, blood flow impairment was in relation with a long and difficult procedure to try to optimise the catheter positioning (avoiding the cystic artery) with possibly a sub-optimal catheter position and potential diffuse spasm (± microthombi) C Second simulation performed 24 h later, using blood flow preservation recommendations, showing a good concordance between MAA targeting and tumour vascularity, validating the accuracy of the simulation and of MAA-based dosimetry. Comparison of the angiography of the first simulation (D) and the second simulation (E) showing a blood flow impairment in the first simulation F 90Y SPECT/CT after microspheres injection using blood flow preservation recommendations, confirming the accurate targeting
Several recommendations have been outlined to preserve the blood flow and improve the accuracy of the simulation-based dosimetry [1, 10, 15]:
-
Limiting the risk of spasm whenever technically possible avoiding coil embolisation and favouring the use of a floppy catheter
-
Limiting the risk of micro-thrombi occurrence (spending as little time as possible in arteries)
-
Taking care of bifurcation proximity, (at more than 1 cm from catheter tip, whenever technically possible)
-
Slow injection of the microsphere surrogate (over 20 to 30 s)
-
Injecting the surrogate and 90Y-microspheres at exactly the same position, including catheter tip orientation in the arterial tree
Tumour Type and Size
The clinical presentation and behaviour of HCC and mCRC are different.
Usually, TARE is used in HCC more frequently in first line, for a unifocal large lesion (or for several lesions), and HCC are typically highly hypervascularised. In fact, the mean reported percentage of MAA injected uptake by HCC is 32% and can reach more than 90% in large and highly vascularised tumours [16]. Tumour to non-tumour uptake ratios (T/NT) are usually high, with a mean T/NT of 7.2 [16].
Metastases treated by TARE, and especially metastasis from Mcrc, are more frequently multifocal disease, with small lesions, hardly previously treated, and with a variable vascularisation (more often less vascularised than HCC). In fact, in one study, the mean MAA incorporated by lesions was only 1.5% [17] and T/NT is usually lower, with a mean value of only 1.7 as reported in one study [18].
Tumour size has a direct impact on SPECT/CT or PET/CT quantification with a risk of partial volume effect. This is the reason why usually dosimetric evaluation of tumours smaller than 2 cm is not done [12, 19, 20]. Furthermore, the smaller the tumour is, the larger the effect of co-registration error on quantification will be. Tumour size also more than likely has an impact on the dose distribution with a more heterogeneous distribution for large lesions due to a more heterogeneous vascularisation and due to frequent necrosis areas. To overcome this issue, injecting more microspheres using a lower specific activity may be of interest (as it would increase the homogeneity of the dose distribution and radiobiological effect). However, more studies are required, as no data comparing the effect of particle number on tumour distribution and clinical outcome are available.
Prior Therapy
Prior therapy such as chemoembolisation [21] or antiangiogenic drugs [22] may induce arterial disorders and weakness, including spasm, occlusion, dissection, and coagulation disorders. In this situation, reproducibility of the two angiographic procedures may be not optimal. High discordance between MAA and microsphere distribution have already been described in this situation [28]. Prior therapy may also have an impact on safety.
The use of 99mTc Macro-Aggregated Albumin Lung Shunt Evaluation (LSF)
The use of MAA provides an overestimation of lung shunt quantification (and then an underestimation of liver and tumour doses). This is related to the fact that MAA particles are slightly lower in size in comparison with microspheres (90% of MAA particles between 10 and 40μ) and has been definitely demonstrated in a study evaluating LSF based on either MAA or 166Ho microspheres [23].
Correlation between MAA-Based Dosimetry and 90 Y-Based Dosimetry
Many studies have compared the values of absorbed doses based on MAA-based dosimetry and 90Y-based dosimetry with discrepant results, and it remains debated whether discrepancies between Y90-and MAA-based dosimetry relate to surrogacy issues of MAA or rather to the ability to deliver both products at the exact same position and under the exact same conditions.
Several of them found a poor correlation between MAA- and 90Y-based dosimetry in tumours and normal liver tissue. They were mainly carried out in patients with metastatic disease using either resin microspheres [14, 24,25,26] or glass microspheres [13] and most often were biased by several technological issues such as catheter repositioning [24,25,26] or absence of spasm evaluation [14, 14, 24,25,26]. However, more and more recent studies, accurately designed, found a strong correlation between MAA-based dosimetry and 90Y-based dosimetry for resin microspheres [28,29,30,31,32,33] as well as for glass microspheres [12, 20, 28, 29, 32] more frequently for HCCs [1220,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. For metastasis, a strong correlation between MAA- and 90Y-based dosimetry was recently found for tumour as well as for normal liver dose evaluation [29], and a strong correlation for the Whole Normal Liver Dose (WNLD) but a weaker correlation for the Tumour Dose (TD) [33] was found in another study.
Usually, the correlation is higher for the Normal Perfused Liver Dose (NPLD) than for the TD [13, 19, 28, 32, 33]. In one study, the correlation was higher for HCC than for mCRC but equal between glass or resin spheres [29].
Correlation between MAA-Based Dosimetry and Clinical Outcome
Instead of focusing on MAA/90Y correlation, many studies in HCC have evaluated and found a good predictive value of MAA dosimetry for response or overall survival for both glass and resin spheres [8, 16, 35,36,37,38,39,40].
This means, when accurately performed (i.e. avoiding technical issue) from a clinical point of view, MAA-based dosimetry is sufficiently accurate in predicting outcomes, even if some variability exists with regard to the prediction of actual 90Y dosimetry.
Main Dosimetry Results
Hepatocellular Carcinoma (Table 1)
For HCC, the tumouricidally absorbed dose reported based on MAA quantification are between 205 and 257 Gy for 90Y-loaded glass microspheres [8, 16, 35,36,37,38] and between 100 and 120 Gy for 90Y-loaded resin microspheres [39, 40] (Table 1). Study type and level of evidence (LOE) are detailed in Table 1.
In the largest study with 90Y resin microspheres evaluated for response (109 patients, RECIST 1.1), the mean TD for patients with disease control was 121.4 Gy vs only 85.1 Gy for patients with progression, p = 0.0204 [40]. This study [40] is the post hoc dosimetry analysis of the randomised SARAH trial [42].
In the largest study with glass microspheres (130 lesions, 85 patients) [37], the response rate based on European Association for the Study of the Liver (EASL) criteria was 91% for lesion with a TD ≥ 205 vs only 5.5% for a TD < 205, p < 10–3. The false positive rate (corresponding to nonresponding lesions with a TD ≥ 205 Gy) was quite high, e.g. 33.3% for TDs ≥ 205 Gy, and < 260 Gy, and very low, only 3.2%, for TD ≥ 260 Gy (p = 0.0012), in accordance with the fundamental radiobiology law: “the higher the dose above the threshold dose, the more severe the damage”.
An impact on overall survival (OS) has also been demonstrated. With 90Y resin microspheres, a median OS of 14.1 months (95% Confident Interval (CI): 9.6–18.6 months) has been reported for patients with a TD > 100 Gy vs only 6.1 months (95% CI: 4.9–6.8 months) for those with a TD < 100 Gy, p < 0.0001 [40]. For 90Y glass microspheres, OS was 21 months (95% CI: 15–27 months) for a TD > 205 Gy vs 6.5 months (95% CI: 3–24 months) for a TD < 205 Gy, (p = 0.0052), with a relative risk (RR) of death of 2.35 (95% CI:1.26–4.4) for a TD < 205 Gy (p = 0.0053) [37]. Impact of TD on OS was even higher for patients with portal vein thrombosis (PVT) with a RR of death of 6.99 (95% CI: 1.98–24.39) for a TD < 205 Gy (p = 0.0025) [37].
90Y SPECT/CT or PET/CT dosimetry confirmed a strong dose response correlation with a quite similar range of tumouricidal absorbed dose for HCC than based on MAA quantification, also with a tendency to be a little bit lower between 61 and 110 Gy for resin [19, 43, 44] and between 160 and 200 Gy for glass [45, 46] with only one study [19] providing a significantly lower value of 188 Gy for glass (using anatomical segmentation and a dose point Kernel algorithm).
No dosimetry results are currently available for 166Ho microspheres, but data should be available soon.
Metastases of Colorectal Carcinoma (Table 2)
For mCRC and 90Y microspheres, most of the results have been provided using 90Y resin microspheres and all using 90Y PET dosimetry. Then, developing personalised dosimetry based on MAA dosimetry seems more challenging. It has to be underlined that for mCRC, FDG-PET/CT was used in all studies for tumour segmentation [27, 47,48,49]. Study type and LOE are detailed in Table 2.
The results of studies with 90Y resin microspheres are quite homogeneous with the tumouricidal dose described between 39 and 60 Gy depending on the study [27, 47, 49].
In the study of Willowson et al. (22 patients, 63 lesions), the mean TD was 51 ± 19 Gy for responding lesions and 26 ± 19 Gy for nonresponding ones, p < 0.0001 [27]. The threshold TD of 50 Gy was predictive of response with a sensitivity of 91% [27]. In the study of Levillain et al. (24 patients, 57 lesion), the threshold TD of 39 Gy was predictive of response with a sensitivity of 80% and a specificity of 95% [47]. This value was also associated with an improvement in OS: the median OS was 5 months for a TD < 3 9 Gy versus 13 months for a TD > 39 Gy, p = 0.0012, [47].
With 90Y glass microspheres, only one study was reported (85 lesions, 24 patients) [49]. Based again on 90Y PET, the threshold tumour dose predicting a response with the greatest accuracy was 139 Gy (sensitivity 77%, specificity 89%), while a dose of 189 Gy predicted response with a specificity of 99% (but with a sensitivity of 45%) and was associated with better overall survival.
Two studies are available with 166Ho microspheres [50, 51]; in the largest one (133 lesions, 40 patients) [50], the mean tumoural dose was 88% higher in patients with response than in patients with progressive disease (p = 0.011) and a mean tumour dose higher that 90 Gy was associated with a significant better overall survival (HR 0.16; 95%CI, 0.06–0.511; p = 0.0031).
Dosimetry and Liver Toxicity (Table 3)
The maximal liver-tolerated dose is more complex to define, as several confounding factors have to be taken into account, such as toxicity definition (including grade and reversibility), treatment line, underlying liver disease and severity, and hepatic reserve (non-irradiated liver) [1, 8]. NPLD and WNLD can be evaluated as proposed by Chiesa et al. [52] (then taking into account the hepatic reserve).
A specific syndrome has been described by Sangro et al. [53]; the Radioembolisation-induced liver disease (REILD) defined by the occurrence during the first 2 months after TARE of a rise in bilirubin over 51 µmol/L and/or ascites, in the absence of tumour progression or bile duct dilatation.
Studies evaluating liver dose and liver toxicities were mainly performed on HCC patients. Their type and LOE are detailed in Table 3.
Using 90Y resin spheres in HCC patients, Strigari et al. [44] evaluated the NPLD (90Y SPECT/CT, Monte Carlo dose voxel kernel and BED). A NPLD of 52 Gy was predictive of a 50% probability of ≥ G2 liver toxicity in patient treated by a whole liver approach (absence of hepatic reserve). Allimant et al. [43] evaluated the area under the dose-volume histograms (AUDVHs) as dose parameter (90Y PET/CT dosimetry, MIRD approach). Area under dose-volume histograms (AUDVHs) for the normal perfused liver was significantly higher for the patients with liver toxicity (REILD as defined by Sangro et al.) versus those without, respectively, 78.91 Gy versus 53.84 Gy, p = 0.04. In a mixed population of patients without underlying cirrhosis, including 71% of patients with mCRC, the mean NPLD was 36.7 Gy for patients with REILD versus only 25.7 Gy for those without REILD, p = 0.02 [53].
Using 90Y glass spheres, Chiesa et al. [52] evaluated the WNLD (MAA-based dosimetry, MIRD approach). A WNLD of 75 Gy was predictive of a 15% probability of liver decompensation (any liver decompensation, irrespective of its severity and eventual reversibility). This limit has been updated based on the bilirubin level with a WNLD limit of < 50 Gy if bilirubin is > 1.1 mg/dL and < 90 Gy if bilirubin is < 1.1 mg/dL [54]. In another study [35], the NPLD was evaluated (MAA-based dosimetry, MIRD approach). Neither NPLD nor hepatic reserve alone were correlated with severe clinical permanent liver toxicity (Common Terminology Criteria for Adverse Event (CTCAE) V3, G ≥ 3). Only the association of a NPLD > 100 Gy with a hepatic reserve < 30% correlated with severe permanent liver toxicity (p = 0.032). NPLD has been directly evaluated with 90Y PET/CT in one study (Monte Carlo approach) in 27 patients with HCC and seven with liver metastasis [55]. An NPLD threshold of 54 Gy was predictive of more than 50% liver toxicity probability (toxicities of grade 2, laboratory test taken into account).
For PVT patients, another parameter beyond the normal liver dose had a major impact on safety: PVT targeting [35,36,37]. Indeed, in two studies, NPLD evaluated either alone or associated with a low hepatic reserve, was not associated with liver toxicity for PVT patients; in this situation, the only parameter strongly associated with liver toxicity was the absence of MAA-PVT targeting [34, 37].
For 166Ho microspheres, the maximal tolerated dose has been defined in a phase one escalation dose study and is 60 Gy to the whole liver [56].
Cumulative liver dose has never been evaluated; this a limit of all those studies for patients with bilobar disease who received two sequential treatments (separated by 1–2 months), and liver-tolerated dose is still a challenge in this situation.
The use of hepatobiliary scintigraphy using 99mTc-mebrofenin has been proposed to evaluate liver function before TARE [57]. Indeed, hepatobiliary scintigraphy allows for an absolute evaluation of liver function as well as for regional evaluation of liver function (i.e. separate evaluation of the right liver and left liver function). Sufficient liver function is characterised by a 99mTc-mebrofenin clearance of at least 2.69 ml/min. Based on this evaluation, it should possible to define prior treatment if the function of the untreated liver will be sufficient (99mTc-mebrofenin clearance of the untreated liver has to be > 2.69 ml/min). However, the situation is not so simple. Indeed, liver function has been sequentially evaluated with 99mTc-mebrofenin before and after unilobar TARE [58]. A strong reduction in the function of the treated liver was observed but unexpected and transient decrease in the function of the untreated lever was observed until month 2 (minus 20%) before reaching a significant increase in month 3.
Nevertheless, evaluation of liver function could be helpful in difficult situations, i.e. when baseline liver function of the patient is limited, when the hepatic reserve is low, or in case of bilobar treatments.
Dosimetry and Contralateral Liver Hypertrophy
Few studies have evaluated dosimetry and contralateral liver hypertrophy.
It has to be considered that after TARE, contralateral liver hypertrophy could occur in relation with injuries of the normal perfused liver and/or with tumour response (related to a production of cytokine and growth factors) [59]. It is mandatory to keep in mind this concept of two different target tissues as threshold doses inducing tumour damage or normal perfused liver damage.
Only one retrospective study evaluated the potential impact of MAA-based dosimetry and future remnant liver (FLR) hypertrophy in HCC patients treated with 90Y glass microspheres [59]. FLR hypertrophy ≥ 10% was significantly more frequent for patients with a high NPLD (≥ 88 Gy, i.e. in 92.2% for a NPLD ≥ 88 Gy versus 65.7% for a NPLD < 88 Gy, p = 0.032). FLR hypertrophy ≥ 10% was also significantly more frequent for patients with a TD ≥ 205 Gy and a tumour volume (TV) ≥ 100 cm3 in patients with initial FRL < 50%. Finally, FLR hypertrophy ≥ 10% was seen in 83.9% of the patients with either a NPLD ≥ 88 Gy or a TD ≥ 205 Gy for tumours larger than 100cm3 (85% of the cases), versus only 54.5% (p = 0.0265) for patients with none of those parameters.
Recently, a retrospective study evaluated post-procedural 90Y PET/CT dosimetry in a population of 56 mixed patients (HCC, cholangiocarcinoma and metastases) treated with 90Y resin microspheres [60]. NPLD and normal perfused liver V30 (fraction of perfused liver receiving at least 30 Gy) were correlated to FLR hypertrophy especially for patient with a low initial FRL (< 30%). A normal perfused liver V30 of 49% was predictive of an increase of the FLR to a value ≥ 40% with the best accuracy (sensitivity 80%, specificity 81.8%, accuracy 80.9%).
For the first time, those two studies supply dosimetry data that could be helpful to personalise treatment with the objective to stimulate FLR.
Personalised Dosimetry
Multi-Compartment Personalised Dosimetry
Multi-compartment personalised dosimetry is necessarily based on the evaluation of the simulation-based dosimetry.
The clinical impact of personalised dosimetry has been evaluated only with 90Y-loaded glass microspheres and for HCC.
A first retrospective study compared 20 patients treated with a standard dosimetry approach (80–150 Gy to the liver) and 51 who received a personalised dosimetry approach targeting more than 205 Gy to the tumour [35]. The RR was significantly improved: 86% with personalised dosimetry versus 55% with standard dosimetry, p = 0.001, without impacting the safety profile.
In a second retrospective study focused on PVT patients, including PVT targeting evaluation, personalised dosimetry targeting > 205 Gy to the tumours provided good clinical results in a retrospective study (41 patients) with an OS of 20.2 months for patients with both a tumour dose > 205 Gy and a good PVT targeting on MAA (good candidates) versus only 3 months (p < 0.001) if one or both criteria were absent (poor candidate) [36].
In the study of Spreafico et al., the main dosimetry endpoint was to provide the supposed maximal tolerated dose for the whole normal liver, whatever the tumour dose [61]. In this study, 120 HCC patients with PVT were treated using this concept with 90Y-loaded glass microspheres. The median OS reported was 14.1 months (CI 95%: 10.7–17.5) comparing favourably with a median OS of 10.4 (CI 95%: 7.2–16.6) and 10 months (CI 95%: 7.7–10.9) previously reported in studies without personalised dosimetry [53, 54], but comparing less favourably to with results achieved with personalised dosimetry based on targeting > 205 Gy to the tumour (median OS 18 months) [36].
Finally, level 1 evidence of the clinical impact of personalised dosimetry has been provided with the multicentre randomised phase II study DOSISPHERE-01 [62], still using glass microspheres. Sixty HCC patients with large lesions (mean size about 11 cm) and often with PVT (68%) were randomised to receive 90Y glass microspheres either with personalised dosimetry (targeting a TD > 205 Gy and if possible > 250–300 Gy) or standard dosimetry (targeting 120 ± 20 Gy to the perfused liver). The response rate was strongly increased with personalised dosimetry (71% dosimetry vs 36% for standard dosimetry, p = 0.0074) as OS, with a median OS more than double with personalised dosimetry (26.6 months vs 10.7 months for standard dosimetry, p = 0.0096).
Uni-Compartment Personalised Dosimetry: Radiation Segmentectomy and Lobectomy
Personalised dosimetry based on uni-compartment dosimetry is also an option when the fraction of untreated liver is sufficient to preserve safety, with the concepts of radiation segmentectomy and radiation lobectomy. In this situation, the goal is to maximise the mean absorbed dose to the perfused volume (without evaluation of the TD and NLD) with the objective to maximise the TD. By definition, with 90Y-loaded glass microspheres, providing more than 150 to the perfused volume is a treatment intensification [35].
Radiation segmentectomy was described first by Riaz et al. in 84 patients with lesions < 5 cm treated by 90Y glass microspheres [63]. Using a high median segmental absorbed of 524 Gy, no clinically relevant liver toxicity was reported [63]. A segmental dose > 400 Gy has been identified to be predictive of complete pathological response in 100% of the cases in 45 HCC patients with a median tumour size of 2.5 cm (min: 1.3 cm–max: 8 cm) [64].
For radiation lobectomy, the DOSISPHERE trial supports the use of lobar dose > 150 Gy if the whole liver dose is < 150 Gy for a Child Pugh A patient and mainly unilobar treatment [62]. Indeed, based on central dosimetry evaluation, 52% of the treated patients received a PLD > 150 Gy, RR was 86.2% versus 33.3% for patients with a PLD, respectively > 150 Gy versus < 150 Gy (p < 10–3), and median OS was 30.8 months (95% CI: 11.7-not reach) vs 10.3 months (95% CI: 5.6–17.6) for patients with a PLD respectively > 150 Gy versus < 150 Gy, p = 0.0064 (unpublished data from DOSISPHERE study).
International Dosimetry Recommendations
Based on published data, the use of personalised dosimetry is now recommended by four international recommendation papers for HCC and other tumours, regarding 90Y resin microspheres [65,66,67], 90Y glass microspheres [15, 67], and 166Ho microsphere [67]. Main recommendations for 90Y-loaded microspheres (where more data are available) are summarised in Table 4.
Conclusion
During the last decade, many studies have provided sustained data confirming the dose response relationship awaited with TARE. Tumouricidal doses have been identified for the different devices and for different tumour types. Liver-tolerated doses have also been described also more complex to identify. Personalised dosimetry has been developed and is now recommended by several international expert groups. Level-one evidence for the major impact of personalised dosimetry on response and overall survival in HCC is now available, bringing a new standard approach for TARE in clinical practice as for trial design. New metrics based on voxel dosimetry will likely contribute to further improvements in TARE dosimetry.
References
Garin E, Rolland Y, Laffont S, Edeline J. Clinical impact of (99m)Tc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with (90)Y-loaded microspheres. Eur J Nucl Med Mol Imag. 2016;43:559–75.
Braat AJAT, Prince JF, van Rooij R, Bruijnen RCG, van den Bosch MAAJ, Lam MGEH. Safety analysis of holmium-166 microsphere scout dose imaging during radioembolisation work-up: a cohort study. Eur Radiol. 2018;28(3):920–8.
SIR-Spheres® Y-90 resin microspheres [package insert]. St Leonards, New South Wales, Australia: Sirtex Medical Pty Ltd; December 2019. https://www.sirtex.com/au/clinicians/package-insert/
TheraSphere™ Yttrium-90 Glass Microspheres [package insert]. Marlborough, MA; Boston Scientific Corporation; 1999. https://btgplc.com/BTG/media/TheraSphere-Documents/PDF/10093509-Rev8_English-searchable.pdf
Walrand S, Hesse M, Chiesa C, Lhommel R, Jamar F. The low hepatic toxicity per Gray of 90Y glass microspheres is linked to their transport in the arterial tree favoring a nonuniform trapping as observed in posttherapy PET imaging. J Nucl Med. 2014;55(1):135–40.
Pasciak AS, Abiola G, Liddell RP, Crookston N, Besharati S, Donahue D, et al. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur J Nucl Med Mol Imag. 2020;47(4):816–27. https://doi.org/10.1007/s00259-019-04588-x
Chiesa C, Maccauro M, Romito R, Spreafico C, Pellizzari S, Negri A, et al. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90)Y microspheres: the experience of the National Tumour Institute of Milan. Quart J Nucl Med Mol Imag. 2011;55:168–97.
Garin E, Lenoir L, Rolland Y, Laffont S, Pracht M, Mesbah H, et al. Effectiveness of quantitative MAA SPECT/CT for the definition of vascularized hepatic volume and dosimetric approach: phantom validation and clinical preliminary results in patients with complex hepatic vascularization treated with yttrium-90-labeled microspheres. Nucl Med Commun. 2011;32(12):21245–55.
Garin E, Rolland Y, Edeline J. 90Y-loaded microsphere SIRT of HCC patients with portal vein thrombosis: high clinical impact of 99mTc-MAA SPECT/CT-based dosimetry. Semin Nucl Med. 2019;49(3):218–26.
Garin E, Palard X, Rolland Y. Personalised dosimetry in radioembolisation for HCC: impact on clinical outcome and on trial design. Cancers (Basel). 2020;12(6).
Kafrouni M, Allimant C, Fourcade M, Vauclin S, Guiu B, Mariano-Goulart D, et al. Analysis of differences between (99m)Tc-MAA SPECT- and (90)Y-microsphere PET-based dosimetry for hepatocellular carcinoma selective internal radiation therapy. EJNMMI Res. 2019;9(1):62.
Haste P, Tann M, Persohn S, La Roche T, Aaron V, Mauxion T, et al. Correlation of Technetium-99m Macroaggregated Albumin and Yttrium-90 Glass microsphere biodistribution in hepatocellular Carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J Vasc Interv Radiol. 2017;28:722–30.
Wondergem M, Smits ML, Elschot M, de Jong HW, Verkooijen HM, van den Bosch MA, et al. 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med. 2013;54:1294–301.
Salem R, Padia SA, Lam M, Bell J, Chiesa C, Fowers K, et al. Clinical and dosimetric considerations for Y90: recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imag. 2019;46(8):1695–704.
Garin E, Laurence Lenoir L, Yan Rolland Y, Julien Edeline J, Habiba Mesba H, et al. 99mTc-MAA SPECT/CT based dosimetry accurately predicts tumour response and survival in HCC patients treated with 90Y-loaded glass microspheres: preliminary results. J Nucl Med. 2012;53(2):255–63.
Van de Wiele C, Maes A, Brugman E, D’Asseler Y, De Spiegeleer B, Mees G, et al. SIRT of liver metastases: physiological and pathophysiological considerations. Eur J Nucl Med Mol Imag. 2012;39(10):1646–55.
Flamen P, Vanderlinden B, Delatte P, Ghanem G, Ameye L, Van Den Eynde M, et al. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with Yttrium-90 labeled resin microspheres. Phys Med Biol. 2008;53(22):6591–603.
d’Abadie P, Walrand S, Hesse M, Annet L, Borbath I, Van den Eynde M, et al. Prediction of tumour response and patient outcome after radioembolization of hepatocellular carcinoma using 90Y-PET-computed tomography dosimetry. Nucl Med Commun. 2021;42(7):747–54.
Martin M, Hocquelet A, Debordeaux F, Bordenave L, Blanc JF, Papadopoulos P, et al. Comparison of perfused volume segmentation between cone-beam CT and (99m)Tc-MAA SPECT/CT for treatment dosimetry before selective internal radiation therapy using (90)Y-glass microspheres. Diagn Interv Imag. 2021;102(1):45–52.
Sueyoshi E, Hayashida T, Sakamoto I, Uetani M. Vascular complications of hepatic artery after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2010;195(1):245–51. https://doi.org/10.2214/AJR.08.2301.
Elice F, Francesco RF. Side effects of anti-angiogenic drugs. Thromb Res. 2012;129(Suppl 1):S50–3. https://doi.org/10.1016/S0049-3848(12)70016-6.
Elschot M, Nijsen JF, Lam MG, Smits ML, Prince JF, Viergever MA, et al. (99m)Tc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with (166)Ho-microspheres. Eur J Nucl Med Mol Imag. 2014;41(10):1965–75.
Ulrich G, Dudeck O, Furth C, Ruf J, Grosser OS, Adolf D, et al. Predictive value of intratumourtumoural 99mTc-macroaggregated albumin uptake in patients with colorectal liver metastases scheduled for radioembolization with 90Y-microspheres. J Nucl Med. 2013;54(4):516–22.
Ilhan H, Goritschan A, Paprottka P, Jakobs TF, Fendler WP, Todica A, et al. Predictive Value of 99mTc-MAA SPECT for 90Y-Labeled Resin Microsphere Distribution in Radioembolization of Primary and Secondary Hepatic Tumours. J Nucl Med. 2015;56(11):1654–60.
Kucuk ON, Soydal C, Araz M, Ozkan E, Aras G. Evaluation of the response to selective internal radiation therapy in patients with hepatocellular cancer according to pretreatment (99m)Tc-MAA uptake. Clin Nucl Med. 2013;38(4):252–5.
Willowson KP, Hayes AR, Chan DLH, Tapner M, Bernard EJ, Maher R, et al. Clinical and imaging-based prognostic factors in radioembolisation of liver metastases from colorectal cancer: a retrospective exploratory analysis. EJNMMI Res. 2017;7(1):46.
Gnesin S, Canetti L, Adib S, Cherbuin N, Silva Monteiro M, Bize P, et al. Partition model-based 99mTc-MAA SPECT/CT predictive dosimetry compared with 90Y TOF PET/CT posttreatment dosimetry in radioembolization of hepatocellular carcinoma: a quantitative agreement comparison. J Nucl Med. 2016;57(11):1672–8.
Jadoul A, Bernard C, Lovinfosse P, Gérard L, Lilet H, Cornet O, et al. Comparative dosimetry between 99mTc-MAA SPECT/CT and 90Y PET/CT in primary and metastatic liver tumours. Eur J Nucl Med Mol Imag. 2020;47(4):828–37.
Richetta E, Pasquino M, Poli M, Cutaia C, Valero C, Tabone M, et al. PET-CT post therapy dosimetry in radioembolization with resin 90Y microspheres: comparison with pre-treatment SPECT-CT 99mTc-MAA results. Phys Med. 2019;64:16–23.
Rhee S, Kim S, Cho J, Park J, Eo JS, Park S, et al. Semi-Quantitative Analysis of Post-Transarterial Radioembolization (90)Y microsphere Positron Emission Tomography Combined with Computed Tomography (PET/CT) images in advanced liver malignancy: comparison with (99m)Tc Macroaggregated Albumin (MAA) Single Photon Emission Computed Tomography (SPECT). Nucl Med Mol Imag. 2016;50(1):63–9.
d’Abadie P, Walrand S, Hesse M, Amini N, Lhommel R, Sawadogo K, et al. Accurate non-tumourtumoural 99mTc-MAA absorbed dose prediction to plan optimized activities in liver radioembolization using resin microspheres. Phys Med. 2021;89:250–7.
Jafargholi Rangraz E, Tang X, Van Laeken C, Maleux G, Dekervel J, Van Cutsem E, et al. Quantitative comparison of pre-treatment predictive and post-treatment measured dosimetry for selective internal radiation therapy using cone-beam CT for tumour and liver perfusion territory definition. EJNMMI Res. 2020;10(1):94.
Skanjeti A, Magand N, Defez D, Tordo J, Rode A, Manichon AF. Selective internal radiation therapy of hepatic tumours: morphologic and functional imaging for voxel-based computer-aided dosimetry. Biomed Pharmacother. 2020;132: 110865.
Garin E, Lenoir L, Edeline J, Laffont S, Mesbah H, Poree P, et al. Boosted selective internal radiation therapy with 90Y-loaded glassmicrospheres (B-SIRT) for hepatocellular carcinoma patients: anew personalized promising concept. Eur J Nucl Med MolImag. 2013;40:1057–68.
Garin E, Rolland Y, Edeline J, Icard N, Lenoir L, Laffont S, et al. Personalized dosimetry and intensification concept with 90Y-loaded glass microsphere radioembolization induce prolonged overall survival in hepatocelluar carcinoma patients with portal vein thrombosis. J Nucl Med. 2015;56(3):339–46.
Garin E, Rolland Y, Pracht M, Le Sourd S, Laffont S, Mesbah H, et al. High impact of macroaggregated albumin-based tumour dose on response and overall survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microsphere radioembolization. Liver Int. 2017;37(1):101–10.
Ho CL, Chen S, Cheung SK, Leung YL, Cheng KC, Wong KN, et al. Radioembolization with 90Y glass microspheres for hepatocellular carcinoma: significance of pre-treatment 11C-acetate and 18F-FDG PET/CT and post-treatment 90Y PET/CT in individualized dose prescription. Eur J Nucl Med Mol Imag. 2018;45:2110–21.
Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70(5):994–9.
Hermann AL, Dieudonné A, Ronot M, Sanchez M, Pereira H, Chatellier G, et al. SARAH Trial Group. Relationship of tumour radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90Y in the SARAH study. Radiology. 2020;296(3):673–84.
Kao YH, Hock Tan AE, Burgmans MC, Irani FG, Khoo LS, Gong Lo RH, et al. Image-guided personalized predictive dosimetry by artery-specific SPECT/CT partition modeling for safe and effective 90Y radioembolization. J Nucl Med. 2012;53(4):559–66.
Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–36.
Allimant C, Kafrouni M, Delicque J, Ilonca D, Cassinotto C, Assenat E, et al. Tumour targeting and three-dimensional voxel-based dosimetry to predict tumour response, toxicity, and survival after Yttrium-90 resin microsphere radioembolization in hepatocellular carcinoma. J Vasc Interv Radiol. 2018;29:1662–70.
Strigari L, Sciuto R, Rea S, Carpanese L, Pizzi G, Soriani A, et al. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: radiobiologic considerations. J Nucl Med. 2010;51:1377–85.
Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Prospective trial using internal pair-production positron emission tomography to establish the Yttrium-90 radioembolization dose required for response of hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;101:358–65.
Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumour dose response after (90)Y-radioembolization with glass microspheres using (90)Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018;102:451–61.
Levillain H, Duran Derijckere I, Marin G, Guiot T, Vouche M, Reynaert N, et al. (90)Y-PET/CT-based dosimetry after selective internal radiation therapy predicts outcome in patients with liver metastases from colorectal cancer. EJNMMI Res. 2018;8(1):60.
Van den Hoven AF, Rosenbaum CE, Elias SG, de Jong HW, Koopman M, Verkooijen HM, et al. Insights into the dose response relationship of radioembolization with resin 90Y-microspheres: a prospective cohort study in patients with colorectal cancer liver metastases. J Nucl Med. 2016;57(7):1014–9.
Alsultan AA, van Roekel C, Barentsz MW, Smits MLJ, Kunnen B, Koopman M, et al. Dose-response and dose-toxicity relationships for yttrium-90 glass radioembolization in patients with colorectal cancer liver metastases. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.120.255745.
van Roekel C, Bastiaannet R, Smits MLJ, Bruijnen RC, Braat AJAT, de Jong HWAM, et al. Dose-effect relationships of 166Ho Radioembolization in colorectal cancer. J Nucl Med. 2021;62(2):272–9.
Bastiaannet R, van Roekel C, Smits MLJ, Elias SG, van Amsterdam WAC, Doan D, et al. First evidence for a dose-response relationship in patients treated with 166Ho Radioembolization: a prospective study. J Nucl Med. 2020;61(4):608–12.
Chiesa C, Mira M, Maccauro M, Spreafico C, Romito R, Morosi C, et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: development of an individualized treatment planning strategy based on dosimetry and radiobiology. Eur J Nucl Med Mol Imag. 2015;42:1718–38.
Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, et al. Liver disease induced by radioembolization of liver tumour; description and possible risk factors. Cancer. 2008;112(7):1538–46.
Chiesa C, Mira M, Bhoori S, Bormolini G, Maccauro M, Spreafico C, et al. Radioembolization of hepatocarcinoma with 90Y glass microspheres: treatment optimization using the dose-toxicity relationship. Eur J Nucl Med Mol Imag. 2020;47(13):3018–32.
Chan KT, Alessio AM, Johnson GE, Vaidya S, Kwan SW, Monsky W, et al. Hepatotoxic dose thresholds by positron-emission tomography after Yttrium-90 radioembolization of liver tumours: a prospective single-arm observational study. Cardiovasc Intervent Radiol. 2018;41(9):1363–72.
Smits ML, Nijsen JF, van den Bosch MA, Lam MG, Vente MA, Mali WP, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 2012;13(10):1025–34.
Braat MNGJA, de Jong HW, Seinstra BA, Scholten MV, van den Bosch MAAJ, Lam MGEH. Hepatobiliary scintigraphy may improve radioembolization treatment planning in HCC patients. EJNMMI Res. 2017;7(1):2. https://doi.org/10.1186/s13550-016-0248-x.
Allimant C, Deshayes E, Kafrouni M, Santoro L, de Verbizier D, Fourcade M, et al. Hepatobiliary Scintigraphy and Glass 90Y Radioembolization with Personalized Dosimetry: Dynamic Changes in Treated and Nontreated Liver. Diagnostics (Basel). 2021;11(6):931. https://doi.org/10.3390/diagnostics11060931 (PMID: 34064296).
Palard X, Edeline J, Rolland Y, Le Sourd S, Pracht M, Laffont S, et al. Dosimetric parameters predicting contralateral liver hypertrophy after unilobar radioembolization of hepatocellular carcinoma. Eur J Nucl Med Mol Imag. 2017;45(3):392–401.
Grisanti F, Prieto E, Bastidas JF, Sancho L, Rodrigo P, Beorlegui C, et al. 3D voxel-based dosimetry to predict contralateral hypertrophy and an adequate future liver remnant after lobar radioembolization. Eur J Nucl Med Mol Imag. 2021;48(10):3048–57.
Spreafico C, Sposito C, Vaiani M, Cascella T, Bhoori S, Morosi C, et al. Development of a prognostic score to predict response to Yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J Hepatol. 2018;68(4):724–32.
Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17–29.
Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol boil Phys. 2011;79:163.
Gabr A, Riaz A, Johnson GE, Kim E, Padia S, Lewandowski RJ, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imag. 2021;48(2):580–3.
Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC, Leung TWT, et al. Patient selection and activity planning guide for selective internal radiotherapy with Yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys. 2012;82:401–7.
Levillain H, Bagni O, Deroose CM, Dieudonné A, Gnesin S, Grosser OS, et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imag. 2021;48(5):1570–84.
Weber M, Lam M, Chiesa C, Konijnenberg M, Cremonesi M, Flamen P, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds Writing group. Eur J Nucl Med Mol Imaging 2021, in press.
Funding
Not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Etienne Garin is consultant for Boston Scientific and reports receiving a grant, personal fees, and non-financial support from Boston Scientific. Boris Guiu is consultant for Boston Scientific declares that he has no conflict of interest. Julien Edeline reports receiving a grant from Boston Scientific during the conduct of the study; personal fees from Boston Scientific, Bayer, Roche, Eisai, Merck Sharpe & Dohme, AstraZeneca and Ipsen; grants and personal fees from Bristol Myers Squibb; and non-financial support from Amgen, outside the submitted work. Yan Rolland reports receiving a grant from Boston Scientific.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garin, E., Guiu, B., Edeline, J. et al. Trans-arterial Radioembolization Dosimetry in 2022. Cardiovasc Intervent Radiol 45, 1608–1621 (2022). https://doi.org/10.1007/s00270-022-03215-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03215-x