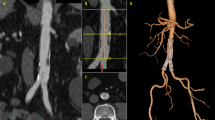
Abstract
Objective
To evaluate feasibility, efficacy and overall functional success of image fusion guidance during laser-assisted in situ fenestration of aortic stent graft (LISFAS) for endovascular repair of complex aortic aneurysm (complex-EVAR) in a prospective study.
Methods
Between September 2016 and July 2018, 20 patients were included and treated with LISFAS for complex-EVAR. Aortic aneurysms were either para-renal (n = 15) or thoraco-abdominal (n = 5) with 57 mm [first quartile: 54; third quartile: 68] median aneurysm diameter in 69 years [68;78] patients. All interventions were performed using the same angiographic system and 3D image fusion software for overlying pre-intervention CTA on per-intervention 2D fluoroscopy with cone-beam CT images to display target vessels ostia.
Results
LISFAS for complex-EVAR with image fusion was performed in all patients, and no endovascular intervention required conversion to an open aortic repair. LISFAS of all target vessels was feasible in 18 patients (90%); 48 fenestrations out of 50 were performed successfully. Two fenestrations failed for renal arteries in two patients. Median ischemic times were as follows: 34 min [25;43] for superior mesenteric artery; 69 min [56;83] for left renal artery; 73 min [36;102] for right renal artery; and 93 min [89;96] for the celiac trunk. Median intervention and fluoroscopy times, iodinated contrast volume and X-ray exposure were 180 min [150;180], 74 min [64;87], 80 mL [59;113] and 338 Gy.cm2 [259;495], respectively. Efficacy was found in 17 patients (85%) on one-week follow-up CTA: Two patients had type 1 and 3 endoleaks, respectively, that were successfully embolized. Overall functional success was 90%. Median hospitalization stay was 9 days [8, 17]. The 30-day safety analysis was 90% (n = 2 deaths) owing to an undetermined cause and to bowel ischemia after low flow in intensive care unit.
Conclusions
LISFAS using image fusion was feasible, efficient and overall functionally successful for complex-EVAR in this preliminary study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular repair of complex aortic aneurysm (complex-EVAR), involving renal and/or visceral branches, based on chimney grafts (CEVAR) or fenestrated/branched (FEVAR/BEVAR) grafts techniques, is an option for vascular repair [1]. The use of custom-made complex endografts as fenestrated/branched grafts is limited to patients with suitable anatomy as well as by production time [1]. For patients unfit for open repair or for conventional complex-EVAR, new options as in situ fenestration of aortic stent grafts (ISFASs) have been described.
Various techniques of ISFAS have been reported as mechanical, such as wires and hollow needles, or physical, including laser and radiofrequency perforation [2]. At the abdominal aortic level, iliac and renal fenestrations were described with mechanical perforation in few cases [3, 4]. A preclinical study showed feasibility of endovascular laser fenestration of endograft (LISFAS) for renal artery [5]. Then, LISFAS was described for different endovascular aortic repair purposes such as revascularization of subclavian artery in thoracic aneurysm and aortic arch branches in dissection or wall hematoma [6, 7]. LISFAS may be promising for endovascular repair of complex abdominal aortic aneurysm (C-AAA), particularly in case of non-suitable anatomy for conventional endovascular repair and in symptomatic aneurysm. Le Houerou et al. [8] described the use of this technique for complex-EVAR in 16 patients. The latter needed a preliminary intervention for stent placement in target artery to display perforation site. The drawbacks of this previous separate session for image guidance purposes are the risk of dissection, thrombosis or stent misplacement (that could further jeopardize LISFAS) and the additional cost of time, radiation and devices.
LISFAS with image fusion guidance using pre-intervention computed tomography angiography (CTA) images represents a new approach for complex-EVAR as described by Touma et al. [9] in a first case report. Image fusion guidance has been described to display a 3D road map onto 2D live fluoroscopy and reduce the use of contrast media and X-ray exposure for complex-EVAR [10, 11]. Therefore, image fusion may facilitate LISFAS by displaying the origin of target vessel ostia. The aim of this study was to investigate feasibility, efficacy and overall functional success of LISFAS with image fusion guidance for complex-EVAR.
Materials and Methods
This study follows the Society of Vascular Surgery Guidelines on the care of patients with an abdominal aortic aneurysm [12]. Institutional review board approval was obtained for this study, and all patients or relatives signed informed consents.
Study Population
In this single-center prospective study, between September 2016 and July 2018, all patients who benefited of LISFAS for complex-EVAR were determined by a multidisciplinary review board team including anesthesiologists, angiologists, vascular surgeons, radiologists and interventional radiologists. Inclusion criteria for complex-EVAR were as follows: (1) C-AAA (para-renal, juxtarenal and thoraco-abdominal aortic aneurysms); (2) high risk of open surgical repair as described by the “Haute Autorité de Santé” (the French counterpart of the Food and Drug Administration) and reported [13]; (3) non-suitable aortic neck anatomy for standard endovascular repair: neck < 10 mm length or > 34 mm diameter based on instruction for use [14, 15]; (4) renal, celiac and mesenteric arteries with anatomy unsuitable for custom-made FEVAR or BEVAR or for CEVAR because of target vessel number, size, location and angles and/or the presence of a previous endograft; (5) patients unfit for custom-made BEVAR or FEVAR due to the urgent need for repair in case of progression on CTA or symptomatic aneurysm; (6) type 1a endoleak in case of previous abdominal aortic endograft; (7) aneurysm secondary to type B aortic dissection. Exclusion criteria were as follows: (1) contraindications to iliac and/or brachial approach due to occlusive disease; (2) unstable atheromatous arterial lesions with risk of embolization [16]; and (3) external iliac diameter < 7 mm or > 24 mm.
Pre-intervention Imaging
All patients underwent pre-intervention multi-detector CT imaging with contrast injection in our institution or elsewhere within 3 months prior to the intervention. Pre-intervention CTAs were evaluated on a diagnostic 3D workstation in order to measure the extent of the aneurysm and to determine the strategy of endovascular repair (sizing of the endograft, number of target vessels for stent placement) [17,18,19,20,21]. CTAs were also used to generate road mapping guidance (further described). In our institution, CTAs protocols are described in detail elsewhere [10].
Image Fusion Guidance
All interventions were performed using the same angiographic system (Allura Xper FD20, Philips Healthcare, Best, The Netherlands) with commercially available software, equipped with 3D volumetric image reconstruction, and image fusion technique (XperCT). All the steps of image guidance are described in detail elsewhere [10]. Briefly, immediately before intervention, the pre-intervention CTA images were loaded into a dedicated 3D workstation (XtraVision Release 8, Philips Healthcare) to be registered with the per-intervention unenhanced cone-beam computed tomography (CBCT) images. Same interventional radiologists (AA and BB with, 25 and 8 years of experience, respectively) who afterward performed the intervention manually registered the CTA and the CBCT images in less than 5 min. The whole volume rendering technique (VRT) of CTA was used to create a 3D road map overlaid on the 2D fluoroscopy [10].
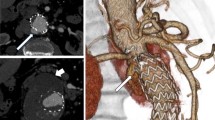
VRT overlay provided the projection of the target vessel on its entire length associated with target ostia vessel landmark represented as a ring (Fig. 1). It was used to select the optimal C-arm angulation during endovascular navigation. The generated 3D road map was synchronized with the C-arm/table positions in order to provide live update and to match the 2D-fluoroscopy at any C-arm/table angle, position and magnification. The image fusion accuracy was assessed at the beginning of each intervention with the control of the correct registration between the two image sets when the catheter was placed into the left renal artery.
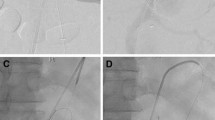
This is a case of an in situ laser fenestration for two target vessels (superior mesenteric and right renal arteries); in this case, landmarks were placed at the ostia places of the celiac trunk, the superior mesenteric artery and the right renal artery. (The left kidney was atrophic and non functional.) First, the Aptus catheter was placed in front of the superior mesenteric artery based on image fusion coronal and sagittal views and the guide wire was placed into the target vessel after perforation, here the superior mesenteric artery (A). A cutting balloon was placed to enlarge the hole (B) followed by a balloon dilatation. A long sheath was placed into the target vessel (C) before the stent placement (D) and deployment (E). The Aptus catheter was then placed in front of the right renal artery on frontal and sagittal views before perforation (F)
Intervention
All endograft deployments and stent placements were performed under general anesthesia. The team performing the interventions included two vascular surgeons (PD and MM) and two interventional radiologists (HK, VT).
With image fusion guidance, aortic endograft component (Endurant, Medtronic, Dublin, Ireland) was first deployed. A catheter (Aptus Heli-FX, Medtronic, Dublin, Ireland) was used to guide the laser probe and maintained its 90° angle with the endograft wall centered by target vessel ostia based on image fusion guidance. LISFAS procedure was a physical retrograde method using a laser perforation device: 0.9 mm Turbo Elite laser catheter with CVX300 system (Spectranetics, Colorado Springs, CL). The fenestration was made with image fusion guidance within 2 to 3 s by laser energy at wavelength of 810 nm with 14 to 18 W as used in the Qin et al. study [6]. After perforation, a 0.014” microcatheter (Pilot0.014, Abbott, Chicago, IL) was used to catheterize the target vessel, always in the same consecutive steps: celiac trunk, superior mesenteric artery and renal arteries. A hole enlargement was done using subsequently a 2.5-mm cutting balloon (Boston Scientific, Marlborough, MA) and a 4- or 5-mm semi-compliant angioplasty balloon (Viatrac, Abbott, Chicago, IL). A 7-Fr 45-cm destination sheath (Destination, Terumo Europe, Leuven, Belgium) was advanced over an exchange 0.035” guide wire (Safe-T-J Rosen, Cook, Bloomington, IN) into the target vessels, allowing the implantation of a covered stent (V12, Atrium Maquet, Hudson, NH) with 1-cm intra-aortic segment.
Before and after stent deployment, 5 ml of iodinated contrast injections (visipaque, iodixanol, GE Healthcare, Cork, Ireland) ensured the correct target vessel catheterization and stent patency. Aorto-bi-iliac component was then deployed followed by the iliac components and, when needed, iliac extensions.
Study Parameters
The main study parameters were feasibility, efficacy and overall functional success. Feasibility was defined as endograft and target vessels successful per-intervention placement and patency at the end of intervention. Efficacy was defined by endograft patency, aneurysm exclusion, target vessels patency and the absence of endoleak on the one-week CTA control. Overall functional success was defined as successful exclusion of the aneurysm without type 1 or type 3 endoleak on the CTA control or severe systemic complication before hospital discharge according to Society of Vascular Surgery criteria [22].
The secondary parameters analyzed other intra-procedural parameters such as ischemic time of each target vessel (recorded from the endograft deployment to the end of the stent placement with patency confirmed with iodinated contrast media injection), total of injected iodinated contrast agent volume, X-ray exposure (dose–area product (DAP)), fluoroscopy time and procedure time. Creatinine levels were recorded at baseline, day 1, day 3 and last value; and lactate level at day 1. The advent of death or major complication during hospital stay and one-month safety data were collected. Moreover, the analysis of the one-month CTA was reported.
Data Analysis
Kolmogorov–Smirnov test was used to determine whether the measurements were normally distributed on R (R Foundation for Statistical Computing, Vienna, Austria). Since all variables were not normally distributed, distribution of continuous variables was described using median and interquartile range (first quartile (Q1)–third quartile (Q3)) and quantitative variables with sum and percentage. The changes in creatinine level across time were done using Kruskal–Wallis rank test. Significant results were tested with pairwise comparison. P value < 0.05 was considered as significant.
Results
Patients and aneurysm characteristics are detailed in Table 1, per-intervention parameters in Table 2 and post-intervention follow-up data in Table 3. LISFAS for complex-EVAR with image fusion was performed in all patients, and no endovascular intervention had to be converted to an open aortic repair. LISFAS of all target vessels was feasible in 18 out of 20 patients (90%). One, two, three or four target vessels were defined for revascularization in, respectively, two, seven, ten and one patients. Vessels were celiac trunk (n = 2), superior mesenteric artery (n = 16), right renal artery (n = 14) and left renal artery (n = 18). Two patients had a planned additional open chimney into the celiac trunk. Forty-eight fenestrations out of 50 were successfully performed (technical success: 96%). Two patients had renal artery catheterization failure due to significant proximal renal artery stenosis.
Five per-intervention immediate complications occurred: (1) one patient underwent an immediate renal artery dissection upon renal stent after stent deployment with partial polar renal occlusion; (2) one patient underwent a superior mesenteric artery dissection successfully treated with two additional stents and; (3) for one patient, Aptus catheter got twisted leading to delay left renal artery fenestration (ischemic time: 102 min) resulting in acute renal failure; this patient died at day 5 (details below); (4) one patient had an acute right lower limb ischemia at the end of the intervention during extubation, requiring urgent thrombectomy; (5) one patient had a minor type 1b endoleak, detected at the final control, which was directly successfully treated with embolization.
The image fusion was manually adjusted at the beginning of the intervention in three patients because of up to 3 mm mismatch of the 3D road map. Image fusion guidance for the LISFAS was not impaired with aortic deformation with rigid guide wire because only the endograft was deployed into the aorta inducing minimal deformation for LISFAS. There was no significant change in creatinine level among patients (p = 0.9) even though one patient needed dialysis during the hospital day. Lactate levels at the day after the intervention remained under 2 mmol/L for all patients except for one patient who had 3 mmol/L.
On the one-week CTA, aneurysms were excluded and all target vessels were patents in 17 patients (efficacy: 85%). The same patient with the unsuccessful LISFAS for a renal artery described above had a minor type 3 endoleak, which was successfully embolized with coils during hospital stay; a second patient had a per-intervention renal dissection with 80% kidney necrosis; a third patient had minor type 1 endoleaks (type 1a + 1b) that were successfully embolized with coils during hospital stay.
Overall functional success was found in 18 patients (90%) because of the advent of two deaths during hospital stay.
The safety of LISFAS during the 48 first hours was 100% and 90% at one month. Three deaths occurred during the follow-up. Two patients died during hospital stay at day 5 and day 48 after the intervention and one patient at home at day 22.
Three major complications occurred in three patients during hospital stay and one-month follow-up. One patient underwent an acute ischemia of the left limb favored by an atheromatous arterial stenosis on day 3 after intervention, which was treated with a crossover bypass, but his health condition declined to death at day 5 due to a presumed mesenteric ischemia related to a low flow. (All stents were patent on the post-intervention CTA.) One patient died during hospital stay of multisystem failure secondary to respiratory failure at day 48. One patient died at home of cardiac failure at day 22. One patient had a hemoperitoneum complication during hospitalization, resolving spontaneously without intervention.
No paraplegia was encountered in our series during hospital stay.
During hospital stay, five secondary interventions were performed: one patient was treated for an acute left lower limb ischemia (patient already noticed above); one patient, with the unsuccessful LISFAS for a renal artery described above, had a type 3 endoleak from the perforations, which was successfully embolized with coils at day 5; one patient had an angioplasty for a right external iliac artery dissection at day 6; one patient had type 1 endoleaks (type 1a + 1b) successfully treated with coils embolization at day 7; one patient had a massive type 2 ilio-lumbar endoleak successfully treated with embolization at day 15.
On the one-month CTA follow-up, no target vessel got occluded, aneurysms diameters remained stable or diminished in size and no advent of endoleak was noticed.
During the follow-up, the patient with the type B dissection had a thoracic extension endoprosthesis at day 15, as initially planned, underwent paraplegia, which partially recovered.
Discussion
LISFAS for complex-EVAR with image fusion was performed in all patients, and no endovascular intervention had to be converted to an open aortic repair. This study assessed feasibility, efficacy and overall functional success of LISFAS for complex-EVAR with image fusion, which were 90%, 85% and 90%, respectively. LISFAS with image fusion was technically feasible for 96% of target vessels and provided acceptable results in patients with challenging need of complex-EVAR. To the authors’ best knowledge, this is the largest cohort study using this technique for complex-EVAR, which is more with the use of image fusion guidance.
The combination of image fusion and LISFAS may help the operator to reduce ischemic time by a precise 3D vision of ostia, which needs to be revascularized. The accuracy of image fusion guidance was demonstrated by Kaladji et al. [23] who claimed precision up to 2.3 ± 1.1 mm on target vessel as visceral vessel location leading to the spread of its use worldwide for complex-EVAR. Feasibility and efficacy and overall functional success of this technique are encouraging and could be applied in various cases as symptomatic or pre-ruptured patient and unfit for conventional complex-EVAR with a personalized in situ complex-EVAR. While comparing renal and visceral cannulations using image fusion guidance in FEVAR in the study of Schwein et al., we reported a higher technical success, 90% versus 81%, probably due to a facilitated fenestration creation at the actual place of target vessel ostia, without any impact of endograft mispositioning [24]. In the same study, fluoroscopy time was 86 min versus 74 min in our study [25].
In our study, the contrast media and fluoroscopy times were comparable to other studies with median results of 80 ml and 74 min versus 57 ml and 61 min as reported by Manunga et al. using FEVAR and BEVAR for complex-EVAR without using image fusion guidance versus 100 ml and 63 min in the Wang et al. study using with similar successful technique [26, 27].
Target vessel ischemic times are the major concern in these types of intervention. In surgical thoraco-abdominal aortic repair, the reported median ischemic times in 171 patients as reported by Kieffer et al. were lower than those in our study: 30 ± 13 min for superior mesenteric artery; 32 ± 16 min for right kidney; and 47 ± 24 min for left kidney. In the same study, the reported spinal cord ischemia was at 5% and the 30-day mortality was 12% [28]. Even though the revascularization times in our series were higher, it did not impact global 30-day mortality. This may be due to the fact that the revascularization times recorded in the study do not represent the ischemic times because the target reperfusion and ischemic time started as soon as the hole enlargement was performed explaining the lack of clinical incidence.
The challenge remains to obtain steerability of perforation device for antegrade fenestration. This was made possible by the use of specific catheter device which allowed a 90° orientation of the laser catheter with the endograft wall despite large diameter of the aorta. In our study, the technique allowed target vessels patency during the one-month follow-up similar to the study of Youssef et al. [29].
In our study, the 30-day mortality was 10% (n = 2) and no hospital stay paraplegia occurred versus 3% mortality and 1% spinal cord ischemia after a C-AAA treatment with FEVAR or BEVAR, as reported by Schanzer et al. [1] on 100 patients. In our study, only one death was directly related to the intervention, while the two others were associated with the poor general condition of high-risk patients (respiratory and cardiac failures) [30].
The study limitations are the limited number of patients, and these preliminary results need to be confirmed with larger cohort and longer follow-up. The main issue for LISFAS technique is the structural damage of the stent graft wall with uncertain long-term concern sealing at the fenestration level. In vitro benchtop evaluations showed minimal change in fenestration size after one year of pulsatile fatigue testing in Dacron Wall [31]. The expanded polytetrafluoroethylene-covered endografts do not seem suitable for laser fenestration because of difficulties to create a laser hole [32].
Long-term surveillance of these endografts after LISFAS is necessary to ensure durability of complex-EVAR. Indeed, LISFAS uses deliberate fabric deterioration of commercially available devices: Type 3 endoleak could develop later. Furthermore, interactions between stent graft and covered stent could provoke stent collapse or breakage, and also a type 3 endoleak.
In conclusion, LISFAS using CTA image fusion was feasible, efficient and overall functionally successful for complex-EVAR in this preliminary study. These encouraging results need to be confirmed with a larger number of patients and longer follow-up.
Abbreviations
- BEVAR:
-
Branched endovascular repair
- C-AAA:
-
Complex abdominal aortic aneurysm
- CBCT:
-
Cone-beam computed tomography
- CEVAR:
-
Chimney endovascular repair
- Complex-EVAR:
-
Endovascular repair of complex aortic aneurysm
- CTA:
-
Computed tomography angiography
- FEVAR:
-
Fenestrated endovascular repair
- ISFAS:
-
In situ fenestration of aortic stent graft
- LISFAS:
-
Laser-assisted in situ fenestration of aortic stent graft
- Q :
-
Quartile
References
Schanzer A, Simons JP, Flahive J, Durgin J, Aiello FA, Doucet D, et al. Outcomes of fenestrated and branched endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66(3):687–94. https://doi.org/10.1016/j.jvs.2016.12.111.
Glorion M, Coscas R, McWilliams RG, Javerliat I, Goeau-Brissonniere O, Coggia M. A Comprehensive review of in situ fenestration of aortic endografts. Eur J Vasc Endovasc Sur. 2016;52(6):787–800. https://doi.org/10.1016/j.ejvs.2016.10.001.
Coscas R, Glorion M, Javerliat I, Goeau-Brissonniere O, Coggia M. In situ fenestration through the contralateral iliac artery to convert an aortouni-iliac into a bifurcated endograft. J Endovasc Ther. 2015;22(3):421–5. https://doi.org/10.1177/1526602815583492.
Kolbel T, Carpenter SW, Diener H, Wipper S, Debus ES, Larena-Avellaneda A. Antegrade in situ stent-graft fenestration for the renal artery following inadvertent coverage during EVAR. J Endovasc Ther. 2013;20(3):289–94. https://doi.org/10.1583/13-4231R.1.
Tse LW, Bui BT, Lerouge S, Salazkin I, Therasse E, Benko A, et al. In vivo antegrade fenestration of abdominal aortic stent-grafts. J Endovasc Ther. 2007;14(2):158–67. https://doi.org/10.1177/152660280701400207.
Qin J, Zhao Z, Wang R, Ye K, Li W, Liu X, et al. In situ laser fenestration is a feasible method for revascularization of aortic arch during thoracic endovascular aortic repair. J Am Heart Assoc. 2017;6(4):e004542. https://doi.org/10.1161/JAHA.116.004542.
Redlinger RE Jr, Ahanchi SS, Panneton JM. In situ laser fenestration during emergent thoracic endovascular aortic repair is an effective method for left subclavian artery revascularization. J Vasc Surg. 2013;58(5):1171–7. https://doi.org/10.1016/j.jvs.2013.04.045.
Le Houerou T, Fabre D, Alonso CG, Brenot P, Bourkaib R, Angel C, et al. In Situ antegrade laser fenestrations during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2018;56(3):356–62. https://doi.org/10.1016/j.ejvs.2018.05.014.
Touma J, Kobeiter H, Majewski M, Tacher V, Desgranges P. Triple in situ antegrade laser fenestration of aortic stent-graft extension using fusion imaging for urgent treatment of symptomatic abdominal aneurysm with type 1 endoleak. Cardiovas Interv Radiol. 2018;41(3):513–7. https://doi.org/10.1007/s00270-017-1837-4.
Tacher V, Lin M, Desgranges P, Deux JF, Grunhagen T, Becquemin JP, et al. Image guidance for endovascular repair of complex aortic aneurysms: comparison of two-dimensional and three-dimensional angiography and image fusion. J Vasc Interv Radiol. 2013;24(11):1698–706. https://doi.org/10.1016/j.jvir.2013.07.016.
Tacher V, Desgranges P, You K, Ridouani F, Marzelle J, Kobeiter H. Feasibility of three-dimensional mr angiography image fusion guidance for endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol JVIR. 2016;27(2):188–93. https://doi.org/10.1016/j.jvir.2015.08.028.
Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2–77. https://doi.org/10.1016/j.jvs.2017.10.044.
Haulon S, Amiot S, Magnan PE, Becquemin JP, Lermusiaux P, Koussa M, et al. An analysis of the French multicentre experience of fenestrated aortic endografts: medium-term outcomes. Ann Surg. 2010;251(2):357–62. https://doi.org/10.1097/SLA.0b013e3181bfda73.
AbuRahma AF, Yacoub M, Mousa AY, Abu-Halimah S, Hass SM, Kazil J, et al. Aortic neck anatomic features and predictors of outcomes in endovascular repair of abdominal aortic aneurysms following vs not following instructions for use. J Am Coll Surg. 2016;222(4):579–89. https://doi.org/10.1016/j.jamcollsurg.2015.12.037.
Verhoeven EL, Vourliotakis G, Bos WT, Tielliu IF, Zeebregts CJ, Prins TR, et al. Fenestrated stent grafting for short-necked and juxtarenal abdominal aortic aneurysm: an 8-year single-centre experience. Eur J Vasc Endovasc Surg. 2010;39(5):529–36. https://doi.org/10.1016/j.ejvs.2010.01.004.
Eesa M, Hill MD, Al-Khathaami A, Al-Zawahmah M, Sharma P, Menon BK, et al. Role of CT angiographic plaque morphologic characteristics in addition to stenosis in predicting the symptomatic side in carotid artery disease. AJNR Am J Neuroradiol. 2010;31(7):1254–60. https://doi.org/10.3174/ajnr.A2078.
Crawford ES, Crawford JL, Safi HJ, Coselli JS, Hess KR, Brooks B, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3(4):389–404.
Bakoyiannis CN, Economopoulos KP, Georgopoulos S, Klonaris C, Shialarou M, Kafeza M, et al. Fenestrated and branched endografts for the treatment of thoracoabdominal aortic aneurysms: a systematic review. J Endovasc Ther. 2010;17(2):201–9.
Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S171–8.
Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg. 1986;3(4):578–82.
Moulakakis KG, Mylonas SN, Avgerinos E, Papapetrou A, Kakisis JD, Brountzos EN, et al. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg. 2012;55(5):1497–503. https://doi.org/10.1016/j.jvs.2011.10.009.
Falkensammer J, Taher F, Uhlmann M, Hirsch K, Strassegger J, Assadian A. Rescue of failed endovascular aortic aneurysm repair using the fenestrated anaconda device. J Vasc Surg. 2017;66(5):1334–9. https://doi.org/10.1016/j.jvs.2017.02.048.
Kaladji A, Dumenil A, Castro M, Cardon A, Becquemin JP, Bou-Said B, et al. Prediction of deformations during endovascular aortic aneurysm repair using finite element simulation. Comput Med Imaging Graph. 2013;37(2):142–9. https://doi.org/10.1016/j.compmedimag.2013.03.002.
Cochennec F, Kobeiter H, Gohel MS, Majewski M, Marzelle J, Desgranges P, et al. Impact of intraoperative adverse events during branched and fenestrated aortic stent grafting on postoperative outcome. J Vasc Surg. 2014;60(3):571–8. https://doi.org/10.1016/j.jvs.2014.02.065.
Schwein A, Chinnadurai P, Behler G, Lumsden AB, Bismuth J, Bechara CF. Computed tomography angiography-fluoroscopy image fusion allows visceral vessel cannulation without angiography during fenestrated endovascular aneurysm repair. J Vasc Surg. 2018. https://doi.org/10.1016/j.jvs.2017.11.062.
Manunga J, Sullivan T, Garberich R, Alden P, Alexander J, Skeik N, et al. Single-center experience with complex abdominal aortic aneurysms treated by open or endovascular repair using fenestrated/branched endografts. J Vasc Surg. 2018. https://doi.org/10.1016/j.jvs.2017.11.093.
Wang SK, Gutwein AR, Gupta AK, Lemmon GW, Sawchuk AP, Motaganahalli RL, et al. Institutional experience with the Zenith Fenestrated aortic stent graft. J Vasc Surg. 2018. https://doi.org/10.1016/j.jvs.2017.11.063.
Kieffer E, Chiche L, Godet G, Koskas F, Bahnini A, Bertrand M, et al. Type IV thoracoabdominal aneurysm repair: predictors of postoperative mortality, spinal cord injury, and acute intestinal ischemia. Ann Vasc Surg. 2008;22(6):822–8. https://doi.org/10.1016/j.avsg.2008.07.002.
Youssef M, Deglise S, Szopinski P, Jost-Philipp S, Jomha A, Vahl CF, et al. A Multicenter experience with a new fenestrated-branched device for endovascular repair of thoracoabdominal aortic aneurysms. J Endovasc Ther. 2018;25(2):209–19. https://doi.org/10.1177/1526602817752147.
Khashram M, Williman JA, Hider PN, Jones GT, Roake JA. Systematic review and meta-analysis of factors influencing survival following abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2016;51(2):203–15. https://doi.org/10.1016/j.ejvs.2015.09.007.
Crawford SA, Sanford RM, Forbes TL, Amon CH, Doyle MG. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J Vasc Surg. 2016;64(1):244–50. https://doi.org/10.1016/j.jvs.2016.03.445.
Verscheure D, Garcia AC, Brenot P, Angel C, Haulon S, Mercier O, et al. In vitro study of anterograde laser fenestration of aortic stentgrafts. Ann Vasc Surg. 2017;44:4–5.
Acknowledgements
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leger, T., Tacher, V., Majewski, M. et al. Image Fusion Guidance for In Situ Laser Fenestration of Aortic Stent graft for Endovascular Repair of Complex Aortic Aneurysm: Feasibility, Efficacy and Overall Functional Success. Cardiovasc Intervent Radiol 42, 1371–1379 (2019). https://doi.org/10.1007/s00270-019-02231-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02231-8