Abstract
Objectives
To evaluate short-term clinical efficacy, complications and possible passive stent expansion of transjugular intrahepatic portosystemic shunt (TIPS) creation using the new controlled expansion ePTFE covered stent (VCX), for portal hypertension complications.
Methods
Between 7/2016 and 3/2018, 75 patients received TIPS using VCX. Thirty-nine patients with VCX dilated with an 8-mm angioplasty balloon underwent computed tomography (CT) study during follow-up and CT data were used to measure stent diameter. The CT measurement technique was validated by ex vivo experiment.
Results
TIPS indications were: refractory ascites (n = 45), variceal bleeding (n = 22), other (n = 8). Mean follow-up was 5.8 months (± 4.5, range 1–20). In 69 patients, TIPS was dilated to 8 mm of diameter reaching the hemodynamic target of a portosystemic pressure gradient (PSG) < 12 mmHg. In six patients, not reaching the hemodynamic target the stent was dilated to 10 mm of diameter during the same session with a final PSG < 12 mmHg. Overall clinical success was achieved in 66/75 (88%) patients (80% in refractory ascites, 95% variceal bleeding, 100% other). Grade II–III encephalopathy was observed in five patients (6%). TIPS revision with stent dilatation to 10 mm was performed in seven patients: in three patients with ascites persistence, without evidence of stent dysfunction and in four patients for stent stenosis. One patient underwent stent reduction. Fourteen patients (18%) died during follow-up of causes not related to TIPS. Five patients (6%) underwent liver transplant. No passive stent expansion was detected by CT measurements.
Conclusion
VCX for TIPS creation retains its diameter over a short-term period and is associated with a good clinical outcome with a reasonably low complication rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) is a well-established procedure for the treatment of complications in cirrhotic patients with portal hypertension such as variceal bleeding and refractory ascites [1]. Despite continued refinements in technique and devices, hepatic encephalopathy (HE) related to diversion of portal blood to the systemic circulation still remains a major drawback of TIPS creation with a reported incidence of 30–50% within the first year after TIPS creation [2,3,4]. Factors associated with post-TIPS HE that could depend directly on stent diameter include a low portal-caval pressure gradient and the volume of blood shunted through the liver [2,3,4,5,6,7]. This has prompted many centers to anecdotally adopt the technique of an underdilation of stent grafts with a nominal diameter of 10 mm at TIPS positioning, with further balloon expansion reserved for cases of insufficient clinical response [1]. However, it has been reported that underdilated stents may passively auto-expand to the nominal diameter after a not predictable period of time, variable from few weeks to some months [8,9,10,11,12] bringing into question the advantages of underdilation for customization of shunt caliber. Recently, a new controlled expansion stent has been introduced in clinical practice (Viatorr Controlled Expansion Endoprosthesis—GORE and Associates, Flagstaff, AZ, USA) (VCX), which allows a lasting diameter control in a labeled diameter range between 8 and 10 mm during implantation to reach a targeted portal pressure gradient. The VCX has been proposed as a potential solution to passive expansion of ePTFE stents [12]. However, very few data are available on this new device [13] and the ability of VCX to assume and maintain the intended diameter has not yet been proven in vivo. Hereby we report our single-center preliminary clinical experience in patients who underwent TIPS creation using the new VCX. We evaluated also whether the VCX dilated with an 8-mm angioplasty balloon retains its diameter, ex vivo and in clinical follow-up, or eventually expand over time using computed tomography (CT) measurements.
Materials and Methods
This single-center observational study was reviewed and approved by the Institutional Research Review Board, and informed consent form was waived. Informed written consent to the TIPS procedure was obtained from all the patients.
Between July 2016 and March 2018, a total of 91 patients underwent TIPS creation for complications of portal hypertension at our institution, using a ePTFE covered endoprosthesis (Viatorr; W.L.GORE and Associates). Refractory ascites, hepatic hydrothorax, and recurrent variceal bleeding were defined according to accepted consensus guidelines [14]. Sixteen patients were not included in the study because they were treated with an ePTFE covered endoprosthesis with nominal diameter of 10-mm followed by 10-mm diameter balloon dilatation (portal cavernoma, massive portal vein thrombosis, Budd–Chiari syndrome with portal vein thrombosis). The clinical study group includes 75 patients treated using the new VCX. Thirty-nine out of seventy-five patients underwent post procedural CT examinations and were included in the imaging study group. Our exclusion criteria for TIPS creation were HE within 3 months prior to TIPS evaluation or previous severe (grade III–IV) HE, multinodular hepatocellular carcinoma, tumoral portal vein thrombosis, ischemic or valvular heart disease, presence of pulmonary hypertension, MELD > 20 and active spontaneous bacterial peritonitis.
TIPS Procedure
All TIPS were performed in an angiographic suite (Innova 4100, GE Medical Systems, Milwaukee, WI, USA) by two faculty interventional radiologists, under general anesthesia. Access to the right or left portal vein branch was achieved with real-time ultrasound guidance using a Colapinto needle (GORE TIPS Set; W.L.GORE and Associates) [15, 16]. Following portal system catheterization the portosystemic pressure gradient (PSG), defined as the difference between the portal pressure and inferior vena cava pressure, was measured [17]. Dilatation of the intrahepatic tract was performed with an 8-mm non-compliant balloon catheter (Mustang; Boston Scientific, Galway, Ireland) subsequently a VCX was deployed followed by 8-mm diameter balloon dilatation. The same 8-mm diameter balloon previously used to dilate the intrahepatic tract was used to dilate the VCX after its deployment. Dilatation was always performed using an inflation device (Encore 26; Boston Scientific) and a pressure of 20 atm, corresponding to the rated burst pressure, was applied. Subsequently PSG was measured again. In patients not reaching the hemodynamic target (< 12 mmHg) the stent was further dilated to 10 mm of diameter during the same session using a 10-mm Mustang balloon with an inflation pressure of 14 atm. All patients were followed-up in the outpatient clinic with clinical, biochemical and Doppler ultrasound (US) evaluation, initially at 1 month after TIPS, then at 3 months, and every 6 months thereafter. TIPS would be revised in case of recurrent variceal bleeding, continued need for paracentesis after more than 3 months after TIPS creation without evidence of HE, and in cases of Doppler findings of TIPS dysfunction.
Ex Vivo Validation of CT Measurement Technique
To test reliability of CT measurements, VCX was analyzed ex vivo. The stent was deployed and dilated with an 8-mm Mustang balloon with an inflation pressure of 20 atm in a 37 °C water bath. After that, the outer stent diameter was measured with a Vernier caliper (precision, 0.02 mm) at the midpoints of the ePTFE graft-lined region and at the unlined portal region. The VCX was then inflected to assume approximately the shape of an implanted stent, kept in the water bath, and scanned by a 64 detector-row CT (VCT 64; GE Medical Systems) in a double oblique orientation of the stent relative to the scanner. Using a commercially available workstation (Advantage Windows 4.6; GE Medical Systems) equipped with multiplanar reconstruction software, double oblique reconstructions were created exactly perpendicular to the long axis of the stent to allow for measurement of the stent diameter on the transverse view at two sections: (1) the midportion of the ePTFE graft-lined region and (2) the unlined portal region. Ten orthogonal slices were reconstructed from each segment and the respective stent diameter extending between the midportion of the metal struts was obtained by a faculty radiologist blinded to the stent dilatation diameter and caliper measurement results. The values measured by CT were compared with the caliper measurements.
Clinical CT Studies
Clinical indications for CT imaging were liver transplant work-up, surveillance, staging or treatment response evaluation of hepatocellular carcinoma, exclusion of procedural complications, and assessment of extent and severity of portal vein thrombosis after TIPS placement. All patients were imaged using a VCT 64 CT scanner (GE Medical Systems). The study protocol included the acquisition of unenhanced images of the entire liver, followed by acquisition of triple-phase contrast material-enhanced images during the hepatic arterial phase, portal venous phase and equilibrium phase.
Image Analysis
CT studies were retrieved from our picture archiving and communication system (Centricity; GE Medical Systems), post-processed on a commercially available workstation (Advantage Windows 4.6; GE Medical System) and reviewed in consensus by two faculty radiologists both blinded to the stents dilatation diameters. Stent diameter was measured between the midportion of the metal struts on contrast-enhanced images acquired during the portal venous phase at the following two sites: (1) at the midpoint of the ePTFE graft-lined region within the intraparenchymal tract (IPT) and (2) close to the portal venous end (PVE) where stent expansion is not influenced by surrounding parenchyma. For both sites, to visualize the stent on true orthogonal short axis images, a coronal image showing the long axis of the stent was obtained first. A perpendicular plane was then traced on this image, and by multiplanar reconstruction, the cross-sectional image of the stent was obtained (Fig. 1). To minimize blooming effect, the window width and level values were set at 1100 Hounsfield unit (HU) and 300 HU, respectively.
Case example of in vivo CT measurement of VCX diameters. A Rendering of VCX stent on follow-up CT scan performed 348 days after TIPS creation demonstrating standard sites of stent diameter measurements at the midpoint of the ePTFE graft-lined region of the stent within the IPT (arrowhead) and close to the PVE (arrow). Multiplanar reconstructed sagittal (B) and coronal (C) CT images with orthogonal plane designation and corresponding transaxial plane with stent diameter measurement (D) at the IPT site
Clinical Data Collection and Statistical Analyses
The following pre-TIPS clinical data were analyzed: age, gender, cause of liver cirrhosis, Child–Pugh score, Model for End Stage Liver Disease (MELD) score, previous HE, previous upper gastrointestinal hemorrhage, radiological (contrast material-enhanced MDCT/MR) evidence of portal vein thrombosis, PSG before and after TIPS creation, and diameter of the stent graft. The Child–Pugh and MELD scores were calculated on the basis of data obtained on the day of TIPS creation.
The variables evaluated during TIPS follow-up included the following: need of paracentesis, long-term need of paracentesis (defined as need of paracentesis after more than 3 months of TIPS creation), overall HE, HE grade III/IV, variceal bleeding, Child–Pugh, MELD, TIPS dysfunction, radiological (contrast material-enhanced MDCT/MR) evidence of recanalization of the portal venous system in patients with portal system thrombosis before TIPS, liver transplantation (censored as alive) and dead.
Patients were followed-up from the date of TIPS creation until the last clinical evaluation, liver transplantation, or death.
Quantitative variables were expressed as mean ± standard deviation, median and range, and qualitative variables as absolute and relative frequencies. Post-TIPS HE and paracentesis rates were assessed for all patients with the Kaplan–Meier method. The association between IPT stent diameter and the time from TIPS creation to CT scan was assessed by linear regression analysis. In a subgroup of patients with two CT scans after TIPS creation, intra-individual stent diameter changes were assessed using paired t test for dependent samples. All tests were two-sided and significance was set at p < 0.05. Data handling and analyses were performed with software (SAS version 9.4; SAS Institute Inc., Cary, NC).
Results
Our study cohort consisted of 75 patients (74 adults, 1 pediatric) treated with TIPS for complications of portal hypertension using the new VCX. Mean follow-up time was 5.8 months (± 4.5, range 1–20). Baseline characteristics of the patients are reported in Table 1. Twenty-nine patients (38%) had radiological evidence of portal vein thrombosis; grade 4 according to Bauer classification (> 75% of luminal occlusion of main portal vein) [18] in 4 cases, grade 1–3 in the remaining 25 patients. Indications for TIPS insertion were prevention of recurrent episodes of gastroesophageal variceal bleeding in patients who had failed endoscopic and/or medical therapy (n = 22), and refractory ascites or refractory hydrothorax (n = 45). Four patients underwent TIPS on the basis of portal system thrombosis alone, unresponsive to anticoagulant therapy, to recanalize the portal vein and maintain transplant waiting list status. Two patients underwent TIPS to treat severe portal hypertension before abdominal surgery (one gastric resection, one colon resection). Two patients underwent TIPS for Budd Chiari syndrome.
Hemodynamic and Clinical Outcomes
TIPS was successfully created in all 75 patients, with no immediate procedural-related complications, such as hemobilia, intrahepatic bleeding or hemoperitoneum, identified within 24 h from TIPS creation.
PSG before TIPS was 15.7 (± 4.8). After TIPS creation PSG was 6.4 (± 2.6). Sixty-nine patients (92%) reached a reduction of the PSG below 12 mmHg with the VCX dilated to 8 mm of diameter. In six patients (8%) not reaching the hemodynamic target the stent was dilated to 10 mm of diameter during the same procedure with a final PSG < 12 mmHg; of note 2 of those patients had grade 4 portal system thrombosis; in a third patient the PSG significantly increased after gastric varices embolization and a 10 mm dilatation was subsequently performed. All patients with pre-TIPS portal system thrombosis underwent contrast material-enhanced CT 1 month after TIPS creation, as routine in our center, showing partially/completely resolved thrombosis in 24 patients (82%), unchanged thrombosis in four patients (14%) and progression in one patient (4%).
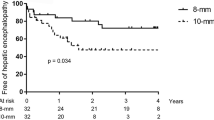
Overall post-TIPS HE was observed in 17 patients (22%) (Fig. 2), grade II–III HE was observed in five patients (6%). Of note 5 out of 17 patients with HE after TIPS had previous episodes of HE before TIPS.
In the subgroup of patients with refractory ascites as indication to TIPS creation, clinical success, defined as disappearance of ascites or significant reduction of paracentesis number, was obtained in 37 patients (80%). Considering patients with more than 3 months of follow-up, clinical success was maintained in 32 patients, 71% (Fig. 3).
In the subgroup of patients with prevention of recurrent episodes of gastroesophageal variceal bleeding as indication one patient had one episode of bleeding secondary to TIPS stenosis; overall clinical success was obtained in 21 patients (95%).
In the subgroup of patients with other indications the overall clinical success was obtained in all patients.
Overall TIPS revision was performed in 8 cases (10%). In detail one patient with a VCX dilated to 8 mm and grade III HE underwent successful stent reduction. Four patients (5%) underwent TIPS revision with dilatation to 10 mm for stent stenosis. Three patients (4%) with ascites persistence after 3 months and no clinical evidence of HE underwent TIPS revision with dilatation to 10 mm although no stenosis were detected both in Doppler US and direct portography; in detail one patient improved his clinical condition after 10 mm dilatation, one patient underwent liver transplant 1 month after revision and one patient had no significant clinical improvement.
After TIPS creation, there were no differences between the baseline mean MELD score and the MELD score at 24 h. Moreover, although there was a mild increase of the MELD score both at 1 and 3 months, this showed a reduction towards baseline MELD at 6 and 12 months; the MELD increase was not associated with a worsened Child–Pugh score (Fig. 4).
Five patients underwent liver transplant from 5 weeks to 6 months after TIPS, in all cases the transplant was not secondary to early liver failure after TIPS. Fourteen patients died during follow-up from 2 to 11 months after TIPS as a result of progressive liver failure (n = 11), HCC progression (n = 1) or unrelated diseases (1 intestinal occlusion, 1 ovarian cancer). All results are summarized in Table 2.
Ex Vivo Measurements of VCX Diameters
The results of caliper measurements of stent diameter at the midpoint of the ePTFE graft-lined region and the unlined portal region were 8.34 and 10.30 mm, respectively. Mean stent diameter measured by CT at the midportion of the ePTFE graft-lined region was 8.32 ± 0.1 mm (median 8.30 mm, range 8.20–8.50). Comparison between CT measurements and the caliper measurement showed nonsignificant differences with a percentage deviation ranging from − 1.92 to 1.68% (p = 0.5086). Mean stent diameter measured by CT at the unlined portal region was 10.21 ± 0.1 mm (median 10.20 mm, range 10.00–10.50). Deviation from caliper measurement ranged from − 1.94 to 2.91% (p = 0.0543).
In Vivo CT Measurements of VCX Diameters
Fifty post-TIPS CT scans were available for 39 out of 75 patients. Mean interval time between VCX placement and first CT scan was 58 ± 43 days (median 40 days, range 0–172). Mean VCX diameters at IPT and PVE site were 8.28 mm ± 0.14 (range 8.00–8.50) and 10.25 mm ± 0.17 (range 10.00–10.60), respectively. There was no association between stent diameter and time after TIPS creation (Fig. 5). Eleven patients (28.2%) underwent two follow-up CT scans after intervention (mean time to first follow-up CT 55 ± 32.4 days, median 34, range 26–126; mean time to second follow-up CT 165 ± 91.4 days, median 147, range 37–348). Mean interval time between CT scans was 110 ± 82.6 days (median 110 days, range 7–279). In this subgroup of patients no significant intra-individual changes of stent diameters were found (mean difference of stent diameter at IPT site between CT scans was 0.02 mm, 95% CI − 0.08 to 0.12 mm, p = 0.6905; mean difference of stent diameter at PVE site was 0.00 mm, 95% CI − 0.15 to 0.15 mm, p > 0.9999) (Fig. 6).
Discussion
Our study reports a single-center preliminary experience in patients who underwent TIPS creation using the new VCX. The results showed that the use of the VCX was associated with a good short-term clinical success with a reasonably low rate of HE and stent revision; moreover, stability over time of VCX diameter was observed by CT measurements. Only few data is currently available on the use of VCX in clinical practice. Praktiknjo et al. [13] showed, in a 3 months follow-up, a reduced readmission rate for sepsis and ascites in 21 patients treated with VCX as compared to a matched cohort of patients treated with bare metal stents and regular covered Viatorr stents. The stent diameter that should be used to create TIPS and treat complications of portal hypertension in cirrhotic patients has not been widely studied. The first study comparing 8 and 10 mm covered stents in TIPS was conducted in 2010 [19]. In this small randomized controlled trial, the probability of remaining free of ascites and variceal bleeding was significantly higher in the 10 mm than in the 8 mm stent group. However, this trial was stopped early due to significantly higher complications in 8 mm group versus 10 mm. In a large retrospective study 10 mm diameter ePTFE covered stent lead to better control of refractory ascites, as compared to an 8 mm stent, without increasing the incidence of HE [20]. In contrast, recently, some studies showed good results in the use of 8 mm covered stent. A large randomized study comparing 8 mm diameter ePTFE covered TIPS stent with a primary pharmacologic approach for the prevention of re-bleeding from esophageal varices showed better results for an elective 8 mm in diameter TIPS in preventing re-bleeding [21]. More recently, a randomized controlled trial, comparing TIPS with 8 and 10 mm covered stents for the prophylaxis of variceal re-bleeding in cirrhotic patients showed that TIPS with 8 mm covered stents did not compromise shunt patency, or influence the efficacy of variceal re-bleeding prevention compared to TIPS with 10 mm stents, but reduced the risk of spontaneous overt HE and the incidence of severe HE [22]. In a recent prospective, non-randomized, study of patients with cirrhosis, the underdilation of covered TIPS, up to 6 mm of diameter, was found to be effective and associated with a low rate of HE [23].
In this “confused” scenario a multi-step dilatation strategy, with modulation of the TIPS diameter, deploying the stent at a diameter smaller than the nominal one as a first approach, has been recommended in the most recent consensus on TIPS management [24]. This approach has been reported in patients with variceal bleeding treated by early TIPS [25] and more recently in patients with refractory ascites [26]. However, this under-sizing is not considered permanent because ePTFE covered endoprosthesis are expected to expand to their nominal diameter after a not predictable period of time [8,9,10,11,12]. To account for this, a modified technique in which a primary externally constrained TIPS shunt, created by deploying a covered stent inside a smaller balloon expandable stent, has recently been proposed as a means to calibrate the PSG to an intended value at TIPS creation or at a later time as needed [27,28,29]. Compared to this technique, the newly released VCX offers the same possibility with a single device. In fact, VCX is identical to the 10-mm Viatorr ePTFE stent graft with the added feature of controlled expansion provided by an outer constraining balloon expandable sleeve on the lined region of the stent graft that allows balloon adjustment of the stent diameter in a labeled range between 8 and 10 mm, maintaining the selected diameter. However, as stated by the manufacturer, these features are based on benchtop data demonstrating less than 0.25 mm diameter expansion by a simulated 10 years period at physiologic portal pressures [30]. Our results showed that at the IPT site the CT measured mean diameter of VCX dilated with an 8-mm balloon was 0.28 mm larger than expected. We hypothesize two possible explanations for this: first, stent diameter within metal struts rather than luminal diameter was measured; second, during stent dilation balloon was inflated up to the rated burst pressure of 20 atm corresponding, according to the balloon specifications, to a diameter of 8.46 mm. However, it should be emphasized that no association was observed between stent diameter and time after intervention and no significant intra-individual size changes were found over time, supporting the stability of VCX diameter, at least within the time frame of the study.
In clinical practice the VCX shows advantages as compared to the standard 8 or 10 mm nominal diameters Viatorr stent grafts: first, the VCX maintains the smallest diameter without significant expansion over time as previously reported in under-sized 10 mm stent; second, it is possible to further expand the 8 mm stent in subsequent procedures in cases of clinical failure, possibility that was precluded with the use of a standard Viatorr covered stent with nominal diameter of 8 mm.
As the main limitation of the study we have only a short-term follow-up and a relatively low number of patients; however, very few data are available on this new device. Other limitations are PSG measurement, in our practice, is performed only during TIPS procedure, under general anesthesia, and not confirmed in a further hemodynamic measurement. In addition, none of the VCXs were dilated to 9 mm of diameter.
In conclusion, in our preliminary experience, the new VCX was safely used in patients with complications of portal hypertension and its use was associated with a good short-term clinical outcome with bleeding and ascites control rates similar to the results reported with standard 10 mm stents, with decreased encephalopathy rate, without passive stent expansion. Further studies, with a longer follow-up, are necessary to confirm our data.
References
Rossle M. TIPS: 25 years later. J Hepatol. 2013;59:1081–93.
Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103(11):2738–46.
Riggio O, Nardelli S, Moscucci F, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liv Dis. 2012;16(1):133–46.
Casadaban LC, Parvinian A, et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(4):1059–66.
Bureau C, Garcia-Pagan JC, Otal P, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469–75.
Bureau C, Pagan JC, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liv Int. 2007;27:742–7.
Sarfeh IJ, Rypins EB. Partial versus total portacaval shunt in alcoholic cirrhosis. Results of a prospective, randomized clinical trial. Ann Surg. 1994;219:353–61.
Pieper CC, Sprinkart AM, Nadal J, et al. Postinterventional passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stents. J Vasc Interv Radiol. 2015;26(3):388–94.
Gaba RC, Parvinian A, Minocha J, et al. Should transjugular intrahepatic portosystemic shunt stent grafts be underdilated? J Vasc Interv Radiol. 2015;26(3):382–7.
Borghol S, Perarnau JM, Pucheux J, et al. Short- and long-term evolution of the endoluminal diameter of underdilated stents in transjugular intrahepatic portosystemic shunt. Diagn Interv Imaging. 2016;97(11):1103–7.
Pieper CC, Jansen C, Meyer C, et al. Prospective evaluation of passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stent grafts—a three-dimensional sonography study. J Vasc Interv Radiol. 2017;28(1):117–25.
Mollaiyan A, Bettinger D, Rössle M. The underdilation of nitinol stents at TIPS implantation: solution or illusion? Eur J Radiol. 2017;89:123–8.
Praktiknjo M, Lehmann J, Fischer S, et al. Novel diameter controlled expansion TIPS (Viatorr CX®) graft reduces readmission compared to regular covered TIPS graft and bare metal graft. J Hepatol. 2017;66:S33–62.
de Franchis R, Baveno V. Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–8.
Miraglia R, Maruzzelli L, Cortis K, et al. Radiation exposure in transjugular intrahepatic portosystemic shunt creation. Cardiovasc Intervent Radiol. 2016;39:210–7.
Miraglia R, Gerasia R, Maruzzelli L, et al. Radiation doses to operators performing transjugular intrahepatic portosystemic shunt using a flat-panel detector-based system and ultrasound guidance for portal vein targeting. Eur Radiol. 2017;27(5):1783–6.
La Mura V, Abraldes JG, Berzigotti A, et al. Right atrial pressure is not adequate to calculate portal pressure gradient in cirrhosis: a clinical–hemodynamic correlation study. Hepatology. 2010;51:2108–16.
Bauer J, Johnson S, Durham J, et al. The role of TIPS for portal vein patency in liver transplant patients with portal vein thrombosis. Liv Transpl. 2006;12(10):1544–51.
Riggio O, Ridola L, Angeloni S, et al. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267–72.
Miraglia R, Maruzzelli L, Tuzzolino F, et al. Transjugular intrahepatic portosystemic shunts in patients with cirrhosis with refractory ascites: comparison of clinical outcomes by using 8- and 10-mm PTFE-covered stents. Radiology. 2017;284(1):281–8.
Sauerbruch T, Mengel M, Dollinger M, et al. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology. 2015;149:660–88.e1.
Wang Q, Lv Y, Bai M, et al. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol. 2017;67(3):508–16.
Schepis F, Vizzutti F, Garcia-Tsao G, et al. Under-dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non-randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol. 2018;16(7):1153–62.e7.
Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liv Dis. 2017;49(2):121–37.
García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;25:2370–9.
Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152(1):157–63.
Farsad K, Kolbeck KJ, Keller FS, et al. Primary creation of an externally constrained TIPS: a technique to control reduction of the portosystemic gradient. AJR Am J Roentgenol. 2015;04:868–71.
Cui J, Smolinski SE, Liu F, et al. Incrementally expandable transjugular intrahepatic portosystemic shunts: single-center experience. AJR Am J Roentgenol. 2018;210(2):438–46.
Rabei R, Mathesovian S, Tasse J, et al. Primary constrained TIPS for treating refractory ascites or variceal bleeding secondary to hepatic cirrhosis. Br J Radiol. 1083;2018(91):20170409.
https://www.goremedical.com/products/viatorr—controlled-expansion. Accessed 28 Jun 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
For this type of study consent for publication is not required.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed Consent
This study has obtained IRB approval from IRCCS-ISMETT and the need for informed consent was waived.
Rights and permissions
About this article
Cite this article
Miraglia, R., Maruzzelli, L., Di Piazza, A. et al. Transjugular Intrahepatic Portosystemic Shunt Using the New Gore Viatorr Controlled Expansion Endoprosthesis: Prospective, Single-Center, Preliminary Experience. Cardiovasc Intervent Radiol 42, 78–86 (2019). https://doi.org/10.1007/s00270-018-2040-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-2040-y