Abstract
Purpose
To evaluate the effectiveness of percutaneous image-guided thermal ablation in achieving local tumor control and pain palliation of sarcoma metastases within the musculoskeletal system.
Materials and Methods
Retrospective review of 64 sarcoma metastases within the musculoskeletal system in 26 women and 15 men (total = 41) treated with ablation between December 2011 and August 2016 was performed. Mean age of the cohort was 42.9 years ± 16.0 years. Two subgroups were treated: oligometastatic disease (n = 13) and widely metastatic disease (n = 51). A variety of sarcoma histologies were treated with average tumor volume of 42.5 cm3 (range 0.1–484.7 cm3). Pain scores were recorded before and 4 weeks after therapy for 59% (38/64) of treated lesions. Follow-up imaging was evaluated for local control and to monitor sites of untreated disease as an internal control. Fifty-eight percent (37/64) were lost to imaging follow-up at varying time points over a year. Complication rate was 5% (3/64; one minor and two major events).
Results
One-year local tumor control rates were 70% (19/27) in all patients, 67% (12/18) in the setting of progression of untreated metastases, and 100% (10/10) in the setting of oligometastatic disease. Median pain scores decreased from 8 (interquartile range 5.0–9.0) to 3 (interquartile range 0.1–4.0) 1 month after the procedure (P < 0.001).
Conclusion
Image-guided percutaneous ablation is an effective option for local tumor control and pain palliation of metastatic sarcomas within the musculoskeletal system. Treatment in the setting of oligometastatic disease offers potential for remission.
Level of Evidence
Level 4, Retrospective Review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcomas are rare, aggressive malignancies derived from mesodermal tissue with approximately 11,000 cases diagnosed in the USA annually [1, 2]. Aggressiveness is largely dependent on histology, site of origin, size, and grade. The overall five-year survival rate is 50–60% for patients with sarcomas of all stages and histologic subtypes [3]. Current treatment algorithms involve resection with wide margins as a mainstay; however, this can be disfiguring [4]. If the margins are insufficient or if the tumor is high grade, adjuvant chemotherapy and/or radiation can be performed. Unresectable gross disease is generally treated with high dose radiation for local control [4, 5].
Depending on the sarcoma characteristics and treatment algorithm, there is a 90% recurrence rate within 5 years [5]. This is partly due to poor response of sarcomas to radiation [6,7,8]. In certain cases, radiation is also contraindicated owing to limited cumulative tolerance of surrounding radiosensitive organs, such as bowel or spinal cord [9], and patients can be excluded from certain systemic chemotherapy trials if radiotherapy is performed [10]. Furthermore, tumor recurrence and metastases to the musculoskeletal system can result in pain from mass effect from the tumor, biochemical stimulation of endosteal nociceptors, and resultant pathologic fracture [11, 12].
There is an emerging role for percutaneous ablation of metastatic disease for local tumor control and pain palliation when radiation therapy is ineffective or contraindicated [10, 13]. Radiofrequency ablation (RFA) is one such modality which uses high frequency alternating current within a needle electrode to create thermal injury and tissue necrosis within surrounding tissue [14]. With osseous metastases, RFA is often used in conjunction with the instillation of cement to prevent or stabilize pathologic fracture [15, 16]. Cryoablation is an alternative modality that causes tissue injury through subfreezing temperatures and can be used in larger, more complex regions of interest, as multiple cryoprobes can be used simultaneously. The ice ball created with cryoablation can be seen on CT, making this option ideal when real-time visualization is required [10, 17].
RFA and cryoablation have shown promise with respect to local tumor control and pain palliation of musculoskeletal metastases from tumors of varying histologies [10, 13, 15,16,17,18]. However, there is a paucity of data on the effectiveness of locally directed treatment of sarcoma metastases within the musculoskeletal system, specifically. The purpose of this study is to evaluate the effectiveness of radiofrequency ablation and cryoablation in local tumor control and pain palliation for this population.
Materials and Methods
Approval by the institutional review board was obtained for retrospective review of a single center institutional database for all image-guided musculoskeletal RFA and cryoablations performed between December 2011 and August 2016 for sarcomas. Informed consent was waived for retrospective study participation.
Selection of the treatment group was made by a multidisciplinary team of medical, radiation, surgical, and interventional oncologists. Treated patients were not candidates for surgery, had malignancies that previously demonstrated radiation resistance, had contraindications to radiation therapy, or had chemotherapy regimens that would have been interrupted by surgery or radiation therapy. Goals of therapy were local tumor control (17%; 11/64), pain control (55%; 35/64), or both (28%; 18/64). Fifty-five percent (29/53) of the lesions treated for pain palliation or both pain and local tumor control were at risk for pathologic fracture. Further details for indications of therapy are summarized in Table 1.
During the study period, 64 metastatic sarcomas in the musculoskeletal system were treated with RFA or cryoablation (Tables 2 and 3). The cohort included 26 women and 15 men (total = 41) with a mean age of 42.9 years ± 16.0 years. The study comprised two subgroups of patients: oligometastatic disease (five or fewer sites of disease; n = 13) and widely metastatic disease (n = 51). There was at least one untreated site in each patient with oligometastatic and widely metastatic disease. Thus, there was no possibility of remission from ablation in these subgroups. Clinical details of patients with widespread and oligometastatic disease are summarized in Table 1.
Ninety-five percent (61/64) of tumors were treated with concomitant chemotherapy (> 50 different chemotherapeutic agents used). The 3 tumors not treated with chemotherapy were from the same patient with widely metastatic hemangiopericytoma; the patient opted for hospice within a month of ablation. Additionally, 23% (15/64) of tumors were treated with concurrent chemoradiation within 3 months of ablation.
A small subset of tumors within this cohort was used in prior publications [10, 13, 16], each of which analyzed a heterogeneous group of tumor histologies. The first study describing pain palliation and local control of musculoskeletal metastases with cryoablation included 19 sarcomas (21%; 19/92) [10]. The second study describing achievability of local control of spinal metastases with radiofrequency ablation and vertebral augmentation included 15 sarcomas (27%; 15/55) [13]. The third study describing feasibility of palliative acetabular radiofrequency ablation and cementoplasty included 2 sarcomas (17%; 2/12) [16]. These publications did not discuss ablation for local control or pain palliation of sarcomas, specifically, and the sample size of sarcomas in this study is much larger (n = 64). Additionally, there was no mention of the implication of treatment of oligometastatic sarcomas, which will be further examined in the discussion.
Radiofrequency Ablation Procedure
RFA was favored in smaller tumors and tumors within the spine (52%; 33/64): Eighty-eight percent (29/33) had widespread metastases, and 12% (4/33) had oligometastatic disease. All procedures were performed under conscious sedation with intravenous midazolam and fentanyl. A 10-gauge introducer cannula (DFINE; San Jose, California) was advanced to the target tumor using CT (36%; 12/33) or fluoroscopic guidance (64%; 22/33). Ablation was performed with the STAR Tumor Ablation System (DFINE), which includes an ablation electrode with an articulated distal segment and two thermocouples located 10 and 15 mm from the ablation electrode that allows for real-time temperature monitoring of the ablation zone dimensions (Fig. 1). The system automatically cuts power to the circuit when the system reaches 50 °C, at which point the dimensions of the ablation zone are 30 × 20 × 20 mm, according to the manufacturer.
RFA of painful supra-acetabular hemangiopericytoma metastasis with subsequent cementoplasty. Prone transaxial CT image demonstrates a lytic metastasis in the right ilium (A). RFA probe with articulating electrode positioned within the osseous sarcoma metastasis (B). Cement instillation at the site of ablation for stability (C)
Cryoablation Procedure
Cryoablation was favored with tumors having extra-osseous soft tissue components or when placement of multiple probes was required for larger lesions (48%; 31/64). Of these, 71% (22/31) had widespread metastatic disease and 29% (9/31) had oligometastatic disease. Procedures were conducted using CT with conscious sedation (87%; 27/31) or general anesthesia (13%; 4/31). Cryoablation was performed with the Endocare Cryocare System (HealthTronics; Austin, Texas) or the Visual-ICE Cryoablation System (Galil Medical; Arden Hills, Minnesota). 10-gauge introducer cannula(e) were advanced to the target, and cryoprobe(s) were placed (range 1 to 18; median: 3) into the tumor 10–15 mm apart to create a continuous cryoablation zone covering the entire region of interest, as well as an additional surrounding 5–10 mm ablative margin [19]. Ablative margin was assumed to be approximately 5 mm within the margin of the hypoattenuating ice ball on CT [20] (Fig. 2). Each ablation consisted of 2 freeze cycles with an intervening and final active thaw. Imaging of the ablation zone was usually conducted at 5 and 10 min intervals in the freeze cycles. This interval shortened when the ablation was next to a critical structure. The duration of the freeze cycle was dependent on whether there was sufficient coverage of the mass and if the ablation zone was close to a sensitive structure. Mean duration of the first freeze cycle, active thaw cycle, and second freeze cycle was 10.6 min (range 5–15 min), 7.1 min (range 5–10 min), and 10.2 min (range 5–15 min), respectively.
Cryoablation of T1 epithelioid hemangioendothelioma metastasis with cervical myelographic hydrodissection for thermal protection. Prone transaxial CT image demonstrates a lytic metastasis involving the T1 spinous process and lamina with pathologic fracture (A). 22-gauge needle tip positioned in the epidural space at C7-T1 level (B). Cryoablation probe within the lytic metastasis. Note the intrathecal contrast allowing for delineation of the spinal cord and thermal protection (C). Low attenuation ice ball encompassing the lytic metastasis and sparing the cord (D)
Thermal Protection
Thermal protection was used in 11% of cases (7/64), including pneumodissection (3/7), hydrodissection (1/7), or both (3/7) through an 18- to 22-gauge needle (Table 2) with the goal to create as much distance as possible between the tumor and a critical structure in close proximity. Somatosensory and motor evoked potentials were measured if the tumor was adjacent to a nerve and the patient was under general anesthesia (5%; 3/64). Warm saline was applied to the skin surface for thermal protection in all cases.
Cementoplasty
Post-ablation cementoplasty of osseous tumors was performed to stabilize or prevent pathologic fractures or if the patient experienced mechanical pain at the time of the procedure. Forty-two percent (27/64) of ablated tumors were treated with adjunctive cementoplasty using the StabiliT System (DFINE) mostly in conjunction with radiofrequency ablation 96% (26/27) (Fig. 1). Cement was instilled the in same introducer cannula(e) used for the ablation probes. Cementoplasty was performed in conjunction with cryoablation in a single case. The cryoablation site was allowed to normalize to room temperature, and cementoplasty was performed to stabilize a left ilium pathologic fracture.
Imaging Assessment of Local Tumor Control
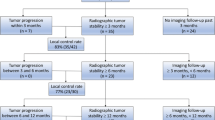
All post-procedure imaging performed at the request of the referring oncologist was retrospectively reviewed. Residual mass-like or nodular enhancement surrounding the ablation zone on first post-treatment imaging within 60 days was considered incomplete ablation or residual disease (5%; 3/64). Any follow-up imaging > 60 days post-ablation was assessed for tumor progression, defined as increased soft tissue tumor, increased enhancement on MRI, or increased osteolysis on CT. For patients with follow-up past 3 months, CT, MRI, and PET/CT imaging was available for 63% (25/40), 58% (23/40), and 38% (15/40) of tumors, respectively. If tumor progression and/or residual disease was seen on initial follow-up imaging, duration of local tumor control was considered to be 0 days. If the patient died, went to hospice, or was otherwise lost to follow-up, the duration of local tumor control was based on their last set of imaging showing no residual or recurrent disease. Post-treatment imaging was also assessed for progression of untreated tumors to serve as an internal control. Thirty-seven treated tumors were lost to follow-up at various time points detailed in Table 4.
Assessment of Pain Palliation
Pain scores were obtained for 59% (38/64) of treated lesions before and 4 weeks after the procedure and were compared using the Mann–Whitney U test. Cryoablation was used for 34% (13/38) of lesions, and 66% (25/38) were treated with RFA. Fifty-eight percent (22/38) were combined with cementoplasty. Additional data including increase in pain medication and increased activity post-therapy were also recorded. Patients treated for pain palliation had a pain score of > 2 based on the Numerical Rating Scale (NRS; 10 point pain score with 10 representing maximal pain) [21] prior to treatment. Successful pain palliation was defined as a decrease in NRS by ≥ 2. Pain increase was defined as any increase in NRS pain score after treatment.
Complications
Patients were evaluated for acute complications after each procedure, which were documented according to the Society of Interventional Radiology (SIR) Classification [22] and the Common Terminology Criteria for Adverse Events v4.0 (CTCAE) [23]. Review of the patient’s electronic medical records was performed to evaluate for delayed complications, such as infection and neurologic injury. Duration of clinical follow-up was recorded for each patient.
Results
Local tumor control results are summarized in Tables 4 and 5.
Local Control of Oligometastatic Disease
One hundred percent (10/10) of treated lesions exhibited local tumor control in the setting of oligometastatic disease. However, 3 of the original 13 ablated lesions were lost to follow-up: 1 at each time point of 3 months, 6 months, and 9 months.
Local Control of Widely Metastatic Disease at 3 months
Twenty-three lesions were lost to follow-up at this time point. The histologies of the 5 tumors that progressed at this time point included leiomyosarcoma, osteosarcoma, Ewings sarcoma (n = 2), and epithelioid hemangioendothelioma. A 0.4 cm3 iliac leiomyosarcoma metastasis with maximum tumor diameter (MTD) of 0.8 cm had initial follow-up 7 months post-ablation showing tumor growth. After re-ablation, local control at 1 year was achieved. An 11.0 cm3 (MTD: 2.5 cm) osteosarcoma acetabular metastasis was incompletely ablated based on initial follow-up imaging at 28 days. The lesion was re-ablated 1 week after initial imaging, and local tumor control was achieved for 9 months. A 0.1 cm3 (MTD: 0.5 cm) thoracic spine epithelioid hemangioendothelioma metastasis showed local progression at 3 months in the setting of progression of untreated metastatic disease. This tumor was retreated with radiation and ablation, which achieved local tumor control for 6 months. A 30.5 cm3 (MTD: 5.8 cm) Ewings sarcoma metastasis within the ilium in the setting of progression of untreated metastases and failed local radiation therapy appeared incompletely ablated on initial follow-up imaging at 44 days, growing to 65.4 cm3 (MTD: 5.8 cm) at 3 months. Residual tumor was present on initial follow-up imaging after a second ablation at 56 days with subsequent tumor growth at 3 months.
Local Control of Widely Metastatic Disease at 6 months
One treated tumor demonstrating stability at 3 months subsequently progressed at 6 months. The tumor was a 2.6 cm3 (MTD: 1.9 cm) Ewings sarcoma metastasis within the acetabulum in the setting of progression of untreated metastases and chemoradiation failure. Nine additional lesions were lost to follow-up at this time point.
Local Tumor Control of Widely Metastatic Disease at 9 months
No additional cases of local tumor progression were identified at 9-month follow-up imaging. However, 2 additional lesions were lost to follow-up at this time point.
Local Tumor Control of Widely Metastatic Disease at 1 year
Two stable tumors at 9 months demonstrated progression at 1 year. One of these tumors was the aforementioned re-ablated acetabular osteosarcoma metastasis (volume: 11.3 cm3; MTD: 3 cm) in the setting of progression of untreated metastases. The other tumor was an osteosarcoma measuring 10.3 cm3 (MTD: 2.6 cm) within the ilium in the setting of progression of untreated metastases.
Pain Palliation
Median pain scores decreased from 8 (interquartile range 5.0–9.0) to 3 (interquartile range 0.1–4.0) 1 month after ablation (P < 0.001). Remaining pain palliation results are summarized in Table 6.
Three patients reported increased pain after therapy. The first was a 65-year-old man with metastatic liposarcoma status post tumor debulking and instrumented posterior spinal fusion of T3–T9 with growing left paraspinal metastatic lesion adjacent to the fused segments. The lesion measured 36.3 cm3 (MTD: 5.5 cm) and was treated with cryoablation with pre- and post-ablation pain scores of 4 and 7, respectively. The second lesion was a 53-year-old woman with metastatic leiomyosarcoma status post-right hemipelvectomy with 114.8 cm3 (MTD: 5.2 cm) recurrent metastatic lesion within the right hemipelvectomy bed involving the ischial tuberosity. The lesion was treated with cryoablation with pre- and post-ablation pain scores of 9 and 10, respectively. Lastly, a 30-year-old woman with metastatic angiosarcoma to the right acetabulum/supra-acetabulum measuring 38.9 cm3 (MTD: 4.5 cm) was treated with RFA and cementoplasty with pre- and post- ablation pain scores of 3 and 4, respectively.
Complications
The complication rate was 5% (3/66) and was graded according to the SIR guidelines [22] and CTCAE [23]. The first complication occurred during an attempted radiofrequency ablation of a sacral tumor adjacent to the left S1 neuroforamen. The patient experienced radicular pain during the procedure despite thermoprotective techniques of combined pneumodissection and hydrodissection. However, the pain subsided when the ablation was stopped with no requirement for overnight observation. This was classified as a SIR Grade A minor or CTCAE Grade 1 minor complication. An additional complication, related to the cryoablation of a rib metastasis, resulted in a hemothorax requiring embolization of an intercostal artery. This was classified as a SIR Grade D major complication or CTCAE Grade 4 life-threatening complication. The final complication was related to superinfection of a right lateral thigh metastasis adjacent to an endoprosthesis treated with cryoablation, requiring operative debridement, antibiotics, and eventual disarticulation of the endoprosthesis. This was classified as a SIR Grade E major complication or CTCAE Grade 3 severe or medically significant but not immediately life-threatening complication.
Discussion
Percutaneous thermal ablation is a safe, effective therapy for achieving local control and pain palliation of sarcoma metastases to the musculoskeletal system. The 1-year local control rate of ablated sarcomas was 70% (19/27) overall, 67% (12/18) in the setting of progression of untreated metastases, and 100% (10/10) in patients with oligometastatic disease. Additionally, 74% of ablated lesions treated for pain showed successful palliative effect at 1-month follow-up. The 5% (3/66) complication rate compares favorably with the morbidity of surgery in many cases and are similar to prior reports of complication rates with ablation [15,16,17, 24, 25].
Aggressive local treatment with the intent of achieving tumor control is performed for varying indications depending on the clinical circumstances. In the setting of incurable widespread metastatic disease, the objective is to minimize pain and other complications related to local disease progression, particularly important in the management of aggressive histologies like sarcomas. In the case of spinal metastases, for example, locally directed treatment could help prevent pathologic fracture or spinal cord compression [15, 17]. Although radiation therapy is the standard of care for palliation of painful musculoskeletal metastases, local tumor control of sarcoma metastases with radiation alone is poor [7, 8]. The current study shows that local control and pain palliation of sarcoma metastases with percutaneous ablation can be achieved and is a viable alternative. In addition, the 66% (19/29) of lesions treated with ablation resulting in pain palliation at 1 month and local control at 3 months is suggestive of a correlation between these two goals of therapy. However, further research with more consistent and extended follow-up is needed to determine whether local control reduces the likelihood of recurrent pain, as well as pathologic fracture or other complications related to local disease progression.
There is growing enthusiasm for aggressive local control of oligometastatic disease, an intermediate state in which tumor cells are able to metastasize to a limited number of sites, but have not yet acquired the capacity to become widely metastatic. It is hypothesized that obliteration of all sites of oligometastatic disease offers the potential for disease remission [18, 25,26,27,28,29,30]. In the present study, percutaneous ablation achieved a local control rate of 100% (10/10). However, indications for treatment were for palliation and/or local tumor control; at least one metastasis within each of these patients was left untreated because the goal of treatment was not disease remission. As a result, disease free interval could not be calculated. Other studies have also shown promising results for local control of visceral metastases with percutaneous ablation, including a retrospective series by Palussiere et al. in which treatment of 47 pulmonary sarcoma oligometastases with radiofrequency ablation achieved a local control rate of 90% during a median follow-up period of 4 years [26]. Thus, despite their uniquely aggressive nature, local tumor control of oligometastatic sarcomas can be achieved with percutaneous ablation, but further research is required to determine if this could translate to disease remission.
This study has several limitations that should be addressed in future research. First, although the cohort exclusively comprised sarcomas, there remained heterogeneity with regard to specific histologies, grades, and stages. Second, there was no standardized follow-up imaging protocol. Patients with local control of their disease are less likely to receive follow-up imaging, as they are more likely to be clinically asymptomatic. An additional forty-two percent (27/64) of treated lesions belonged to patients who died or went to hospice over the course of a year of follow-up, as would be expected in this study population. Thirteen percent (8/64) of treated lesions were lost to follow-up for unknown reasons. Imaging for the internal control of progression of untreated metastases did not always occur at the same interval as imaging follow-up for the treated tumor, as well. This lack of regular follow-up may be a source of underestimation of overall local tumor control rates and in the setting of progression of untreated metastases. Additionally, pain scores were only obtained for 38 of 53 lesions treated with pain palliation as a part of goal of therapy, contributing another source of bias to the pain control aspect of this study and limiting the ability to correlate successful pain control and local control.
Conclusion
Image-guided percutaneous ablation is an effective therapeutic option for local tumor control and pain palliation of sarcoma metastases to the musculoskeletal system. Additionally, treatment in the setting of oligometastatic disease offers potential for remission. A prospective clinical trial is needed to further substantiate these results.
References
Gilbert NF, Cannon CP, Lin PP, Lewis VO. Soft-tissue sarcoma. J Am Acad Orthop Surg. 2009;17:40–7.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29.
Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109.
Davidge KM, Wunder J, Tomlinson G, Wong R, Lipa J, Davis AM. Function and health status outcomes following soft tissue reconstruction for limb preservation in extremity soft tissue sarcoma. Ann Surg Oncol. 2010;17:1052–62.
Mendenhall WM, Indelicato DJ, Scarborough MT, et al. The management of adult soft tissue sarcomas. Am J Clin Oncol. 2009;32:436–42.
Strander H, Turesson I, Cavallin-Stahl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol. 2003;42:516–31.
Tepper JE, Suit HD. Radiation therapy alone for sarcoma of soft tissue. Cancer. 1985;56:475–9.
Kepka L, DeLaney TF, Herman DS, et al. Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2005;63:852–9.
Masucci GL, Yu E, Ma L, et al. Stereotactic body radiotherapy is an effective treatment in reirradiating spinal metastases: current status and practical considerations for safe practice. Expert Rev Anticancer Ther. 2011;11:1923–33.
Wallace AN, McWilliams SR, Connolly SE, et al. Percutaneous image-guided cryoablation of musculoskeletal metastases: pain palliation and local tumor control. J Vasc Interv Radiol. 2016;27:1788–96.
Urch C. The pathophysiology of cancer-induced bone pain: current understanding. Palliat Med. 2004;18:267–74.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93.
Wallace AN, Tomasian A, Vaswani D, Vyhmeister R, Chang RO, Jennings JW. Radiographic local control of spinal metastases with percutaneous radiofrequency ablation and vertebral augmentation. AJNR Am J Neuroradiol. 2016;37:759–65.
Rybak LD, Gangi A, Buy X, La Rocca Vieira R, Wittig J. Thermal ablation of spinal osteoid osteomas close to neural elements: technical considerations. AJR Am J Roentgenol. 2010;195:W293–8.
Wallace AN, Greenwood TJ, Jennings JW. Use of imaging in the management of metastatic spine disease with percutaneous ablation and vertebral augmentation. AJR Am J Roentgenol. 2015;205:434–41.
Wallace AN, Huang AJ, Vaswani D, Chang RO, Jennings JW. Combination acetabular radiofrequency ablation and cementoplasty using a navigational radiofrequency ablation device and ultrahigh viscosity cement: technical note. Skeletal Radiol. 2016;45:401–5.
Tomasian A, Wallace A, Northrup B, Hillen TJ, Jennings JW. Spine cryoablation: pain palliation and local tumor control for vertebral metastases. AJNR Am J Neuroradiol. 2016;37:189–95.
McMenomy BP, Kurup AN, Johnson GB, et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2013;24:207–13.
Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–75.
Georgiades C, Rodriguez R, Azene E, et al. Determination of the nonlethal margin inside the visible “ice-ball” during percutaneous cryoablation of renal tissue. Cardiovasc Intervent Radiol. 2013;36:783–90.
Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure. Pain Pract. 2003;3:310–6.
Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2003;14:S293–5.
National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events v4.0 (CTCAE). U.S. Depart of Health and Human Serv, Nat Inst of Health, Nat Canc Inst, 2009; NIH pub #09-7473.
Bang HJ, Littrup PJ, Currier BP, et al. Percutaneous cryoablation of metastatic lesions from colorectal cancer: efficacy and feasibility with survival and cost-effectiveness observations. ISRN Min Invasive Surg. 2012;2012.
Bang HJ, Littrup PJ, Goodrich DJ, et al. Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation. J Vasc Interv Radiol. 2012;23:770–7.
Palussiere J, Italiano A, Descat E, et al. Sarcoma lung metastases treated with percutaneous radiofrequency ablation: results from 29 patients. Ann Surg Oncol. 2011;18:3771–7.
Nakamura T, Matsumine A, Yamakado K, et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas [corrected]. Cancer. 2009;115:3774–81.
Yamanaka T, Takaki H, Nakatsuka A, et al. Radiofrequency ablation for liver metastasis from gastrointestinal stromal tumor. J Vasc Interv Radiol. 2013;24:341–6.
Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6:8491–524.
Okunieff P, Petersen AL, Philip A, et al. Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol. 2006;45:808–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jack W. Jennings is a paid consultant and is on the medical advisory board and speaker panel for Merit Medical Inc. and Medtronic.
Rights and permissions
About this article
Cite this article
Vaswani, D., Wallace, A.N., Eiswirth, P.S. et al. Radiographic Local Tumor Control and Pain Palliation of Sarcoma Metastases within the Musculoskeletal System with Percutaneous Thermal Ablation. Cardiovasc Intervent Radiol 41, 1223–1232 (2018). https://doi.org/10.1007/s00270-018-1932-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1932-1