Abstract
Purpose
To compare effectiveness of transarterial chemoembolization (TACE) combined with microwave ablation (MWA; TACE–MWA) with TACE alone for treating hepatocellular carcinoma (HCC) tumors ≤5 cm.
Materials and Methods
We reviewed data of 244 patients treated for HCC by TACE–MWA or TACE from June 2014 to December 2015. Median follow-up period was 505 days (TACE–MWA group: 485 days; TACE group: 542 days). Patients were propensity score matched (1:2 ratio); outcomes of TACE–MWA and TACE groups were compared. Primary endpoints were tumor responses, including tumor necrosis rates after initial treatment, tumor responses at 6 months [per modified Response Evaluation Criteria in Solid Tumors (mRECIST)], and time to tumor progression (TTP). Secondary endpoints were overall survival (OS) and re-intervention times.
Results
After initial treatments, tumor necrosis rates were higher in the TACE–MWA group (n = 48; 92.1% [58/63]) than the TACE group (n = 96; 46.3% [56/121]; P < 0.001). At 6 months’ follow-up, the TACE–MWA group had better tumor responses (CR + PR + SD [per mRECIST]: TACE–MWA, 95.8%; TACE, 64.5%; P < 0.001). The TACE–MWA group had better TTP (P < 0.001), but did not significantly differ in OS (P = 0.317). TACE–MWA decreased re-TACE times from 1.90 to 0.52; and re-MWA times from 0.22 to 0.17. In subgroup analysis, TACE–MWA also showed better TTP in patients with tumors ≤3 cm (P < 0.001) and 3–5 cm (P = 0.004).
Conclusions
Compared with TACE, TACE–MWA leads to better responses for HCC tumors ≤5 cm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide [1]. Although surgical resection is broadly recognized as a curative treatment for HCC, most patients were not surgical candidates because of age, poor hepatic functional reserve, high surgical risk, or their own refusal [2,3,4,5].
Transarterial chemoembolization (TACE) is one of the most widely used primary treatments for patients who are not considered eligible for surgery, and is evolving into the standard therapy for such patients [6, 7]. However, many factors, such as feeding arteries and tumor size, can influence the efficacy of TACE. The tumor’s arterial vasculature critically affects TACE efficacy: adequate embolization is relatively easy for tumors with only one feeding artery, but more difficult for tumors with complex blood supplies. As larger arteries usually have more complex feeding arteries, TACE efficacy tends to decrease with tumor size. For large tumors, complete tumor necrosis is difficult to achieve by TACE alone; reportedly 36–100% of such tumors survive after TACE [8,9,10,11,12]. When tumor necrosis is incomplete, the remaining viable tumors can cause local recurrence and distant metastasis.
Microwave ablation (MWA), a repeatable, minimally invasive intervention, destroys focal tumors by direct energy application [5, 13]. When the coagulated zones created by the microwaves completely cover the tumors and a 5-mm safe margin, MWA is a curative, rather than palliative, technique. Combination therapy with MWA after TACE (TACE–MWA) might substantially benefit patients with HCC.
Propensity score matching is a matched control statistical technique based on cases’ propensity scores, which can strengthen causal arguments in observational studies by reducing potential bias owing to nonrandomized study design when large-scale randomized controlled trials are unavailable.
To our knowledge, this study is the first to compare TACE alone with TACE–MWA for tumors ≤5 cm. We retrospectively compared tumor response, overall survival (OS), and re-intervention times after treatment with TACE–MWA or TACE alone, using propensity score matching method. We also evaluated subgroups by tumor size.
Materials and Methods
Study Design
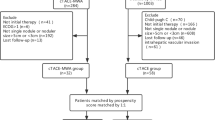
This retrospective cohort study used data from patients with HCC who were treated in our medical center from June 2014 to December 2015. After they underwent TACE (1–3 sessions), we further evaluated 244 patients with HCC who were candidates for subsequent MWA. Patients who received MWA were the TACE–MWA group, and the others were the TACE group. Patients were informed of advantages and disadvantages of the two treatment options, including outcomes, treatment-related morbidities, and costs; treatment decisions were made jointly by patients and their physicians, with full respect for patients’ wishes [14]. We conducted propensity score matching according to Hansen’s theory [15] to match TACE–MWA patients with TACE patients at a 1:2 ratio, and compared their outcomes. Each patient’s follow-up was ended upon his or her death or on November 1, 2016. This study was approved by our institutional review board.
Patient Selection Criteria
We included patients with HCC who (a) had refused or were not suitable for surgical treatment; (b) had ≤3 nodules, ≤5 cm each; (c) had Child–Pugh (CP) class A or B disease; and (d) were treated with TACE–MWA or TACE monotherapy. We excluded patients who (a) had vascular invasion or distant metastases; (b) had secondary malignancies; (c) had histories of other treatments; or (d) were lost to follow-up.
TACE
A 5F sheath was introduced into each patient’s femoral artery using the Seldinger technique and a 5F catheter (Terumo, Tokyo, Japan) used for selected hepatic or superior mesenteric artery angiography, to evaluate tumor location, number, size, and blood supply. A micro-catheter (Terumo, Tokyo, Japan) was then super-selectively inserted into the hepatic lobe or hepatic segmental artery branch, guided by fluoroscopy. After the target artery was catheterized, a 1:1 mixed suspension of iodized oil (1–10 mL; Lipiodol Ultra-Fluide; André Guerbet Laboratories, Aulnay-Sous-Bois, France) and epirubicin (20–40 mg; Pharmorubicin; Pfizer, Wuxi, China) was infused into the artery through the catheter, depending on liver function and tumor size. Finally, gelatin sponge particles (Hanzhou Alc, Hangzhou, China) were infused to embolize the artery until no tumor staining was found after repeat angiography.
TACE–MWA
Patients underwent MWA within 2 weeks after their TACE procedures (as described above). MWA was performed with a microwave ablation system (ECO Corporation, Nanjing, China) at 2450 ± 50 MHz with continuous adjustable power output of 0–150 W, under general anesthesia, using an internally cooled antenna with a homemade grid affixed to the abdominal skin. Plain computed tomography (CT) images were obtained on expiration; reconstructed CT images were generated individually to show tumor shape, tumor location, and optimal puncture trajectory. The equipment was set at the power output and duration recommended by the manufacturer for the tumor’s size. After completing the ablation, a CT scan was performed to verify the coagulation zone and assess possible complications, such as pneumothorax, bleeding, etc. All procedures were performed by the same radiologist, who had 23 years of interventional experience.
Follow-Up
Enhanced liver CT or magnetic resonance imaging (MRI) was performed 1 month after the procedure to verify lipiodol retention inside the tumor and detect viable tumor tissue. If appropriate, TACE or MWA procedures were performed if viable tumors were confirmed, using MWA for nodule-like tumors and TACE for tumors with irregular shapes and/or multiple foci. Follow-up intervals extended to 3–6 months thereafter, if no viable tumor was detected.
At 6 months after treatment [12, 13], we evaluated tumor response using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [16]. Based on mRECIST, we assessed time to tumor progression (TTP), defined as the interval from enrollment to tumor progression, death or last follow-up. Overall survival (OS) was calculated from enrollment to death from any cause, to the last follow-up in censored patients, or to November 2016. We recorded complications from major to minor, according to the Society of Interventional Radiology (SIR) grading system [13].
Statistical Analysis
To minimize effects of potential confounders on selection bias, we conducted propensity scores using binary logistic regression to estimate the probability for which group a patient would be assigned. Independent variables used in the propensity model were determined by previous study [10, 17], including sex, age, tumor number, tumor size, number of previous TACE, Hepatitis B virus (HBV), alpha fetoprotein (AFP), alanine aminotransferase (ALT), aspartate transaminase (AST), and CP class. We used nearest-neighbor matching with no replacement method to accomplish one-to-two matching between the groups, ensuring comparability between patients in the TACE cohort to the TACE–MWA cohort (Stata psmatch2; StataCorp LP, College Station, TX, USA). Fisher’s exact or Chi-square tests for categorical variables and Student’s t test for continuous variables were calculated to compare baseline characteristics. The Kaplan–Meier method and log-rank test were used to evaluate TTP and OS differences between groups. Multivariable Cox proportional hazards models were used to determine effect of TACE–MWA on TTP; hazard ratios (HRs) with 95% confidence intervals (CIs) were then calculated. All tests were two-sided; P < 0.05 was considered significant. All analyses were conducted using SPSS software package version 18.0 (SPSS, Chicago, IL, USA) and STATA 12.0 (StataCorp LP).
Result
Patient Characteristics
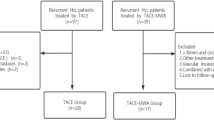
From June 2014 to December 2015, a total of 116 patients with HCC underwent TACE–MWA and 976 underwent TACE in our medical center. We excluded 68 of these TACE–MWA patients and 780 TACE patients, using the criteria shown in Fig. 1. After propensity score matching at a 1:2 ratio, the TACE–MWA group had 48 patients and the TACE group had 96 patients. They did not significantly differ in sex (P = 0.812), age (P = 0.632), tumor number (P = 0.876), tumor size (P = 0.512), number of previous TACEs (P = 0.857), HBV infection (P = 0.282), AFP (P = 0.343), ALT (P = 0.905) or AST (P = 0.834), or CP class (P = 0.316; Table 1).
The median follow-up period was 505 days (TACE–MWA: 485 days; TACE: 542 days). In the first few months of the study, 3 patients in the TACE group died because of tumor progression, on days 119, 126, and 156, respectively.
Therapeutic Process and Treatment Effect
In the TACE–MWA group, after the initial combined therapies, the re-TACE rate was 0.52 ± 0.825 (0 re-TACE: n = 32; 1: n = 8; 2: n = 7; 3: n = 1); and the re-MWA rate was 0.17 ± 0.429 (0 re-MWA: n = 41; 1: n = 6; 2: n = 1).
In the TACE group, after their TACE procedures, the re-TACE rate was 1.90 ± 1.774 (0 re-TACE: n = 25; 1: n = 25; 2: n = 13; 3: n = 12; 4: n = 16; 5: n = 2; 6: n = 2; 9: n = 1); and a post-TACE-MWA rate of 0.22 ± 0.547 (0 MWA: n = 80; 1: n = 12; 2: n = 3; 3: n = 1).
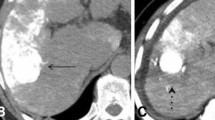
The first follow-up CT or MRI scans showed that 58 tumor nodules (58/63; 92.1%) were completely necrotic in the TACE–MWA group compared with 56 in the TACE-only group (56/112; 46.3%; P < 0.001).
Tumor Response at 6 Months
We compared tumor control between the two groups at 6 months after treatment, using mRECIST criteria [complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD)]. Responses in the TACE–MWA group were CR: 91.7% (44/48); PR: 2.1% (1/48); SD: 2.1% (1/48) and PD: 4.2% (2/48). Responses in the TACE group were CR: 32.3% (30/93); PR: 24.7% (23/93); SD: 7.5% (7/93); and PD: 35.5% (33/93). Total tumor control rates (CR + PR + SD) were TACE–MWA: 95.8%; TACE: 64.5% (P < 0.001).
Tumor Progression and Associated Risk Factors
In the TACE–MWA group, 89.6% (43/48) of the TACE–MWA group did not suffer tumor progression; the median time for tumor progression was therefore not available for the TACE–MWA group. However, it was 398 days in the TACE-only group. Cumulative tumor progression rates were significantly higher in the TACE-only group (0.5 year: 36.5%; 1 year: 47.7%; 1.5 year: 55.7%; 2 year: 57.3%) than in the TACE–MWA group (0.5 year: 4.2%; 1 year: 10.4%; 1.5 year: 10.4%; 2 year: 10.4%; P < 0.001; Fig. 2A).
Multivariate analysis with Cox proportional hazard model revealed that group (HR = 0.144, 95% CI 0.057–0.365, P < 0.001), AFP level (HR = 2.049, 95% CI 0.836–1.335, P = 0.020), and tumor size (HR = 1.069, 95% CI 1.038–1.102, P < 0.001) were associated with tumor progression (Table 2).
Overall Survival
Cumulative survival rates were higher in the TACE–MWA group (0.5 year: 100.0%; 1 year: 91.7%; 1.5 year: 88.5%; 2 year: 88.5%) than in the TACE-only group (0.5 year: 96.9%; 1 year: 87.2%; 1.5 year: 81.1%; 2 year: 77.0%) but not significantly so (P = 0.317; Fig. 2B). Their median survival times are not currently available.
Subgroup Analysis by Tumor Size
In the subgroup with tumors sized ≤3 cm, cumulative TTP rates were significantly higher for the TACE group (0.5 year: 32.0%; 1 year: 38.0%; 1.5 year: 50.2%; 2 year: 52.2%) than for the TACE–MWA group (0.5 year: 0%; 1 year: 3.9%; 1.5 year: 3.9%; 2 year: 3.9%; P < 0.001; Fig. 3A). However, cumulative OS rates did not significantly differ between the two groups (TACE—0.5 year: 100.0%; 1 year: 100.0%; 1.5 year: 96.2%; 2 year: 96.2%; TACE–MWA—0.5 year: 100.0%; 1 year: 100.0%; 1.5 year: 100.0%; 2 year: 95.0%; P = 0.728; Fig. 3B).
Similarly, in the subgroup with tumors sized 3–5 cm, cumulative TTP rates were significantly higher for the TACE group (0.5 year: 43.5%; 1 year: 60.2%; 1.5 year: 60.2%) than the TACE–MWA group (0.5 year: 9.1%; 1 year: 18.2; P = 0.004; Fig. 3C); whereas cumulative OS rates did not significantly differ (TACE—0.5 year: 93.5%; 1 year: 72.1%; 1.5 year: 54.5%; TACE–MWA —0.5 year: 100.0%; 1 year: 81.8%; (P = 0.553; Fig. 3D).
Multivariate analysis with Cox proportional hazard model revealed that (a) for the ≤3-cm subgroup, not undergoing MWA (HR = 0.025, 95% CI 0.002–0.332, P = 0.005) and HBV infection (HR = 0.296, 95% CI 0.093–0.940, P = 0.039) were associated with worse TTP; and (b) for the 3–5-cm tumor subgroup, not undergoing MWA (P = 0.005), tumor number (P = 0.004), tumor size (P < 0.001), HBV infection (P = 0.011), and number of previous TACE procedures(P < 0.05) were independently associated with tumor progression (Table 3).
Safety Evaluation
Several adverse events were noted and graded by the SIR grading system. Post-treatment minor complications (fever, nausea, vomiting, and abdominal pain) occurred in 77.1% (37/48) of the TACE–MWA group and in 81.3% (78/96) of the TACE group. All these effects were transient and relieved before discharge. No major complication related to treatment was observed for either procedure.
Discussion
To our knowledge, this study is the first to compare TACE–MWA and TACE as primary treatments for patients with HCC tumors of ≤5 cm in diameter. Compared with TACE alone, TACE–MWA shows a better tumor response, and may reduce re-interventions.
TACE treatment is a valid option for patients with HCC who are not eligible for curative treatment. According to the Barcelona guideline, TACE should only be used in patients with middle-stage disease, but in clinical practice, TACE also benefits those with early- or advanced-stage HCC, which is called “stage migration strategy” [18, 19]. However, achieving complete response for medium, large, or multiple tumors by TACE is difficult. As microwave techniques have developed in recent years, the area coagulated by MWA has enlarged [9, 20]. Hypothetically, performing MWA after TACE could destroy the tumor completely, leading to better tumor control and survival time.
Patients usually undergo MWA 1–2 weeks after TACE, because patients need time to recover from post-embolization syndrome caused by TACE, and for the lipiodol to clear from the normal liver parenchyma, so as to show the tumor clearly. The TACE–MWA group had notably better results than the TACE-only group, which was expected because MWA has a curative capability. Similar to the results of Yin et al. [12], our study showed 92.1% tumors in TACE–MWA group were completely ablated, compared with 46.3% in the TACE-only group (P < 0.001), and re-TACE time effectively decreased from 1.90 to 0.52. The response rate in our study (CR + PR + SD) was also significantly higher in the TACE–MWA group than that in the TACE group (95.8 vs. 65.2%; P < 0.001). In accordance with Yin’s findings, we also showed that combined TACE–MWA treatment was an independent factor against tumor progression. However, although TACE was associated with shorter TTP, the two groups did not significantly differ in OS (P = 0.317, log-rank test). However, this apparent absence of OS benefit might only reflect our rather short follow-up period; our survival curve did show a nonsignificant trend of better OS in the TACE–MWA group. Furthermore, better tumor control usually predicts better survival expectation [9, 12]. Therefore, delaying the tumor progression through TACE–MWA treatment might lead to better tumor control and patient survival. We did not perform multivariate analysis for OS, because 81.3% (117/144) patients were still alive when the study was terminated.
The main advantage of MWA over radiofrequency ablation (RFA) is producing larger coagulation volume in a shorter time [5]. Tumor size is associated with tumor control, and larger lesions tended to be ablated by MWA rather than RFA; therefore, the similar rates for local tumor control between MWA and RFA reported in an earlier study [21] might reflect treatment selection bias. Abdelaziz [22] also showed RFA was inferior to MWA for tumor control. In the present study, tumor necrosis rates were almost twice as high in the TACE–MWA group (92.1%) as in the TACE-only group (46.3%).
As tumor size is an important factor in tumor control and patient survival time [17, 23], we also divided the cohort into two subgroups by tumor size (≤3 vs. 3–5 cm). Song et al. [17], who combined RFA and TACE, demonstrated survival benefit only in their ≤3-cm tumor subgroup; clinical results of TACE + RFA and TACE monotherapy were similar in for the 3–5-cm tumor subgroup on their research. However, in our study, combined TACE–MWA led to better TTP results for both subgroups, possibly because coagulated zones created by MWA are larger than with RFA. We did not analyze the subgroups’ OS differences because most patients were alive when the study was ended.
This study has a number of strengths. First, it was a well-balanced comparative study that compared the survival benefits of TACE–MWA with those of TACE for treating HCC tumors no larger than 5 cm. Second, it made clear that TACE–MWA induced excellent response rates in the subgroups whose tumors were ≤3, and 3–5 cm, although OS did not significantly differ between these two tumor size subgroups for the limited follow-up period. Our study showed that MWA should be strongly considered in otherwise qualified patients after TACE, and implies that MWA is an option for patients whose tumors were originally larger than 5 cm but shrank effectively after TACE.
Our study has several limitations. First, as the median follow-up period was 505 days, survival data were limited, although the benefits of combined TACE–MWA were confirmed in tumor control. Second, as with most retrospective studies, this research was not randomized in design. For this reason, we used propensity scoring to adjust this potential confounder. Third, all procedures were performed in a single institution, and bias from both the physicians’ experiences and the patient population are possible. Fourth, as no previous study has compared the efficacy of TACE–MWA with TACE in ≤5-cm HCC tumors, we cannot compare our results with other published studies.
Although our findings should be confirmed in prospective randomized controlled trials, our analyses suggest that TACE–MWA offers better tumor control and TTP compared with TACE. Additional MWA should be considered after TACE for tumors ≤5 cm.
References
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55.
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–22.
Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–10.
Bhardwaj N, Strickland AD, Ahmad F, El-Abassy M, Morgan B, Robertson GS, et al. Microwave ablation for unresectable hepatic tumours: clinical results using a novel microwave probe and generator. Eur J Surg Oncol. 2010;36:264–8.
Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–63.
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71.
Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9.
Hashimoto T, Nakamura H, Hori S, Tomoda K, Nakanishi K, Murakami T, et al. Hepatocellular carcinoma: efficacy of transcatheter oily chemoembolization in relation to macroscopic and microscopic patterns of tumor growth among 100 patients with partial hepatectomy. Cardiovasc Interv Radiol. 1995;18:82–6.
Xu LF, Sun HL, Chen YT, Ni JY, Chen D, Luo JH, et al. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28:456–63.
Terzi E, Piscaglia F, Forlani L, Mosconi C, Renzulli M, Bolondi L, et al. TACE performed in patients with a single nodule of hepatocellular carcinoma. BMC Cancer. 2014;14:601.
Dai WC, Cheung TT, Chok KS, Chan AC, Sharr WW, Tsang SH, et al. Radiofrequency ablation versus transarterial chemoembolization for unresectable solitary hepatocellular carcinomas sized 5–8 cm. HPB (Oxford). 2015;17:226–31.
Yin X, Zhang L, Wang YH, Zhang BH, Gan YH, Ge NL, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849.
Ahmed M, Technology Assessment Committee of the Society of Interventional R. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25:1706–8.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Hansen BB. Full matching in an observational study of coaching for the SAT. J Am Stat Assoc. 2004;99(467):609–18.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Song MJ, Bae SH, Lee JS, Lee SW, Song do S, You CR, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med. 2016;31:242–52.
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–59.
Liu C, Liang P, Liu F, Wang Y, Li X, Han Z, et al. MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperth. 2011;27:654–62.
Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379–84.
Abdelaziz A, Elbaz T, Shousha HI, Mahmoud S, Ibrahim M, Abdelmaksoud A, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014;28:3429–34.
Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN, et al. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014;109:1234–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all of the authors, the corresponding author states that there are no conflicts of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chen, QF., Jia, ZY., Yang, ZQ. et al. Transarterial Chemoembolization Monotherapy Versus Combined Transarterial Chemoembolization–Microwave Ablation Therapy for Hepatocellular Carcinoma Tumors ≤5 cm: A Propensity Analysis at a Single Center. Cardiovasc Intervent Radiol 40, 1748–1755 (2017). https://doi.org/10.1007/s00270-017-1736-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1736-8