Abstract
Purpose
To assess the feasibility and safety of transperineal laser ablation (TPLA) for treating benign prostatic hyperplasia (BPH).
Materials and Methods
Institutional review board approval was obtained for this prospective non-randomized trial. Eightteen patients (age 71.7 ± 9.4 years) with urinary symptoms secondary to BPH underwent TPLA under local anesthesia. Under US guidance, up to four 21G applicators were inserted in the prostatic tissue. Each treatment was performed with diode laser operating at 1064 nm changing the illumination time according to prostate size. Primary endpoints were technical success and safety of TPLA. Secondary endpoints included operation time, ablation time, energy deployed, hospitalization time, catheterization time, and change in International Prostate Symptom Score (IPSS), Quality of Life (QoL), peak urinary flow rate (Q max), post-void residual (PVR), and prostatic volume at 3 months. χ 2 and Fisher exact tests were used.
Results
All procedures were technically successful. No complications occurred. Mean operation time was 43.3 ± 8.7 min, mean ablation time 15.9 ± 3.9 min, mean energy deployed 10,522 ± 3290.5 J, mean hospital stay 1.5 ± 0.4 days, and mean catheterization time 17.3 ± 10.0 days. At 3 months, IPSS improved from 21.9 to 10.7 (P < 0.001), QoL from 4.7 ± 0.6 to 2.1 ± 1.2 (P < 0.001), Q max from 7.6 to 13.3 mL/s (P = 0.001), PVR from 199.9 ± 147.3 to 81.5 ± 97.8 (P < 0.001), and mean prostate volume from 69.8 to 54.8 mL (P < 0.001).
Conclusions
TPLA is feasible and safe in the treatment of BPH, providing significant clinical results at 3 months.
Level of Evidence
Case series, Level IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transurethral resection of the prostate (TURP) and open prostate adenomectomy are regarded as the “gold standard” for the surgical treatment of male lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) [1]. This option, however, can be associated with significant complications [2]. Over the last decade, there has been effort to identify new technologies that can replicate the effectiveness of TURP but with an improved safety profile. Actually, green light laser, holmium laser, thulium laser with transurethral approach can be offered as a treatment alternative to TURP [3,4,5]. Other transurethral thermal ablative technologies, such as transurethral needle ablation (TUNA) or transurethral microwaves therapy (TUMT), produced disappointing and not durable results for relieving of clinical symptoms, being proven less effective in producing a symptoms relief compared to TURP [5, 6]. Recently, there has been a burgeoning interest in development of new technologies aimed at a minimally invasive and rapid therapy for patients with LUTS associated with BPH, particularly focusing on different route than transurethral one to deliver treatment, one of the most promising being prostate embolizations [7,8,9,10,11,12,13,14,15].
Ultrasound (US)-guided interstitial laser ablation has been widely applied for the percutaneous treatment of malignant and benign diseases in different body regions [16,17,18]. To the best of our knowledge, use of US-guided interstitial laser ablation for the treatment of BPH has never been reported with transperineal approach. Thus, we designed a prospective study in order to assess the feasibility and safety of transperineal laser ablation (TPLA) for treating BPH.
Methods
Patients
This prospective, non-randomized, single-center pilot trial was approved by the Ethical Committee and Institutional Review Board of our hospital. Patients coming to the Department of Interventional Radiology and Department of Urology at our institution with LUTS caused by BPH were assessed for study eligibility and prospectively enrolled between May 2014 and May 2016. Patients had been assigned to TPLA either they were poor surgical candidates (open prostatectomy or TURP) or did not fit into surgical criteria of conventional transurethral laser prostatectomy. Patients were included if they had LUTS and at least one of the following criteria: (a) male subject >50 years of age who have symptomatic BPH; (b) International Prostate Symptom Score (IPSS) score ≥13; (c) prostate volume ≥30 mL on transrectal ultrasonographic (TRUS) images; (d) peak urinary flow rate (Q max): ≥5 to ≤15 mL/s; (e) post-void residual (PVR) ≥50 mL. Exclusion criteria were: (a) urethral stricture; (b) previous prostate, bladder neck, or urethral surgery; (c) prostate cancer or patients who had a prostate-specific antigen (PSA) value greater than 4 ng/mL; (e) patients with known neurological disorders, e.g., multiple sclerosis, Parkinson’s disease, or known history of spinal cord injury.
Before treatment, all patients completed the IPSS and Quality of Life (QoL) questionnaire and underwent pressure-flow urodynamics to evaluate Q max, transabdominal US to determine PVR, and transrectal US to determine the volume of the prostate. All patients signed a dedicated informed consent.
Transperineal Laser Ablation (TPLA) Treatment
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Before the procedure, routine coagulation tests were performed and included prothrombin time, partial tromboplastin time, and platelet count in accordance with the guidelines used for interventional procedures in other organs, such as liver, kidney, or lung [19]. The patient was placed in the radiological interventional suite in a gynecological position (Fig. 1). A three-way 18-F Foley catheter was inserted and followed by continuous irrigation with saline during and after the maneuver. The TPLA was performed by the interventional radiology and urology team using the combined Echolaser XVG system (Elesta s.r.l. 50041, Calenzano (FI), Italy). Each treatment was performed with patient under conscious sedation by IV of midazolam (3 mg) and with local anesthesia of the superficial tissues of the perineal region and prostate anesthesia by transrectal prostatic block with lidocaine solution 2% (20 mL) [20]. All patients were treated with antibiotic therapy from the previous day and for a variable period after the LA treatment session pertinent to individual patients’ risk profiles. Using a two-plane transrectal ultrasound probe (TRT 33, Esaote, Genoa, Italy), depending on basal volume of the prostate, up to two 21G introducer needles for each lobe were inserted in the para-urethral sites and placed on planes as parallel as possible to the longitudinal plane of the prostate. The system is equipped with a needle guide attachment that allows for a more precise parallel insertion of the needles. For prostate volume <40 mL, two fibers were used, while four fibers were used for prostates larger than 40 mL (Fig. 2A, B). Subsequently, a 300-µm bare flat-tip optical laser fiber was introduced and advanced up to needle tip. The introducer needle was designed to expose the fiber tip of 5 mm. Care was taken to maintain a safety distance of 8 mm from the needle to the outer wall of the urethra and of at least 15 mm from the needle tip to the bladder floor (Fig. 2B). The applicators were inserted one at a time, spaced least 1.5–2.0 cm one to the other. The applicators positions were always confirmed with two-plane US images (Fig. 2C). The optical fibers were then connected with a continuous-wave (CW) diode laser source operating at 1064 nm with four independent devices for their simultaneous firing during the illumination (Echolaser XVG system; Elesta s.r.l. 50041, Calenzano, Italy). Each treatment was performed with a fix power protocol (3 W) changing the illumination time case by case according to prostate size. Each ablation time ranged from a minimum of 400 s to a maximum of 600 s to maintain the total energy applied between 1200 and 1800 J per fiber. Depending on the size of the prostate, from one to two consecutive illuminations were performed with a “pull-back” technique during the same treatment session. Treatment was concluded when the gas forming during the ablation covered the entire desired area or 1800 J per illuminations were reached (Fig. 2D). At the end of the treatment, desametasone (8 mg) was administered intravenously for antiedemigenous and antiphlogistic purposes. After an observation period of approximately 1 h, the patient underwent a transrectal ultrasound with the administration of an echo-amplifying contrast medium (SonoVue, Bracco, Italy) to assess the extent of coagulation zone (Fig. 2E). The patient was kept in the hospital for 2 days and the catheter, in the absence of adverse events, was removed within 2 weeks after the procedure. All procedures were performed by one interventional radiologist (G.P.) with 20 years of experience in interventional radiology and in minimally ablative techniques.
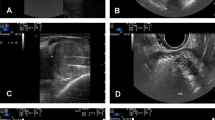
Representative case of large BPH of 110 mL in a man of 65 years of age. A Axial and longitudinal TRUS image before TPLA shows a prostatic gland with large volume at baseline. B Axial TRUS image shows a correct positioning of four devices (two for lobe) at a safe distant from urethral lumen and outer margin of the prostate at the end of targeting. C Longitudinal TRUS images show the fine needles properly spaced between them both in the right (1.6–1.7 cm) and in the left (1.6–1.9 cm) lobe. D Longitudinal US image shows the target area completely covered by the gas at the end of the treatment. E Axial and longitudinal US image during the administration of an echo-amplifying contrast medium (Sonovue) shows an oval hypoenhancing areas in both lobes due to induced coagulation zone by TPLA
Follow-Up Assessment
Primary endpoint of this study was technical success and safety of TPLA. Technical success was defined following standard terminology use for tumor ablation [19] as the ability of completing the treatment according to the protocol. Thus, treatment was considered to be feasible when it was possible to insert all the desired needles and fibers that were preoperative established in the desired zone and to complete the ablation up until the desired endpoint.
The definition of complications was consistent with the standardized terminology and reporting criteria for image-guided tumor ablation proposed by other authors [19, 21]. Major complications, minor complications, and side effects were classified as intraoperative, perioperative (within 24 h), postoperative (within 30 days), and delayed (after 30 days).
Secondary endpoints included: (a) operation time; (b) ablation time and energy deployed; (c) hospitalization time; (d) catheterization time and change in IPSS, QoL, Q max, PVR, and prostatic volume at 3 months. Prostatic volume was measured by transrectal ultrasound.
Statistical Analysis
Analysis was performed using GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA). Continuous variables were expressed as mean ± SD, and categorical variables displayed as frequencies and compared using the χ 2 or Fisher test, as appropriate. A P value of less than 0.05 was considered statistically significant.
Results
Eightteen TPLA procedures were performed to treat 18 patients (mean age 71.7 ± 9.4, range 51–89 years). A median lobe was present in 9 (50%) out of 18 patients. The mean prostate basal volume, mean IPSS, mean QoL, mean PVR, and mean Q max of peak urinary flow are reported in Table 1.
Primary Endpoints
All procedures were technically successful. In 18/18(100%) cases, correct targeting of the prostatic lobes was achieved, and ablation was performed as planned. We used one applicator per lobe in 4/18 cases (22%) while two applicators were used in 14/18 (78%) cases. The procedural details are reported in Table 2. All patients were discharged within 2 days of admission. None of the patients required re-catheterization.
All patients required only therapeutic regimen allowed with minor hospitalization (<48 h), while none of the patients required major therapy, unplanned increase in level of care, or prolonged hospitalization (>48 h) or have had permanent adverse sequelae. None of the patients experienced minor complications and side effects. None of the patients required intensive care, surgical, endoscopic, or radiological interventions. No patient had long catheterization, long-time hospitalization, and perioperative complications such as bleeding, hematuria, or necrosis.
Secondary Endpoints
Comparison of IPSS, QoL, Q max, PVR, and prostate volume at baseline and at scheduled time point of 3 months is reported in Table 1. Statistically significant improvements were seen in IPSS, QoL, PVR (P < 0.001), and Q max (P = 0.001). The average prostate size at 3 months was 54.8 ± 29.8 mL, shoving a 19% reduction in size versus baseline (P < 0.001).
Discussion
The present study is the first to report TPLA in treating patients with LUTS due to BPH. The results of this study demonstrated that TPLA is a feasible and safe procedure for the treatment of patients with LUTS due to BPH. The reported data were comparable with those reported with surgical options [22] and with other new minimally emerging alternative methods [8, 10, 11, 13]. We observed a significant improvement in all parameters at 3 months from baseline values (Table 1). Only the volume reduction in the prostate seems less relevant in comparison with all other values although statistically significant. Based on experience in treating benign lesions in others organ [18], it is possible to speculate that by laser technique the process of volumetric reduction in the treated lesions occurs slowly over time often constantly increasing up to 12 month [18]. In other words, TPLA is not a immediate ablative technique. So, we should expect a significant improvement in prostate volume at longer follow-up. For the same reason, and because we included mainly quite complex patients that were poor surgical candidates, we had a quite long catheterization time. However, as this was our initial experience, we think this should be reduced in the future, also by applying this technique to less complex patients. Further studies focusing on volume reduction over time are necessary to better clarify this issue and particularly to understand if larger ablation zone could be related to a better clinical result.
We proposed TPLA as acronym for this new procedure over the most used laser interstitial thermal therapy (LITT) as we wanted to underline the peculiar aspect of this procedure, which is the transperineal approach.
As regards the adverse events, we simply point out the absence of any type of complication and side effects both intraoperatively, immediate postoperative, perioperative, and delayed. It is likely that the transperineal approach and the use of very small and little traumatic thin applicators are responsible for this low complication rate. Moreover, we used low-energy density to coagulate the tissue of the obstructing prostatic adenoma, with constant real-time US monitoring. The real-time assessment of the gas forming during the treatment in combination with the slow release of energy allowed for a precise monitoring of the procedure, allowing for avoiding ablation of undesired structures.
In our view, the main advantage of TPLA is its ease of use. Thanks to the use of fine needles with this technique it seems relatively easy to perform various ablative treatment phases. The correct positioning of one or two heat sources under US guidance into prostatic lobe is relatively quick and easy (Fig. 2B). Equally easy is to see and follow the ablation process and their therapeutic effects during the procedure and monitor how well the target is being covered by the gas forming during the procedure, and whether any adjacent structures (i.e., urethral lumen or/and outer fibrous capsule) are being reaching by the treatment (Fig. 2C, D). The use of intraprocedural transrectal US guidance with biplane probe allows, when needed, repositioning of applicators during the maneuver based on the physician experience, imaging findings, and the amount of energy planned in advance according to the baseline volume of prostatic lobes. Finally, we found easy to assess the extent of coagulation zone early after ablation with transrectal contrast-enhanced US (Fig. 2E) and considered postoperative contrast-enhanced ultrasound a very useful tool for early result assessment [23]. However, all these relatively easy procedural phases require, in our view, a well-trained operator (interventional radiologist or urologist).
On the basis of experience in the last two decades in treating benign and malignant lesions in other organ [16,17,18] with this low-power diode laser through percutaneous route, we developed this novel application to reduce prostate volume and improve LUTS caused by BPH. Thermal ablations, including diode laser, radiofrequency, and microwaves, have been already used for the treatment of BPH [3, 5, 6]. However, in all the reported experiences, thermal energy was delivered to the prostate gland through the transurethral route. These technologies have not approached the effectiveness or durability of TURP, probably a result of poor resorption of the thermally desiccated tissue and lack of true tissue debulking. By using the transperineal route, the bare laser flexible fibers can be placed directly within prostatic tissue keeping a safe distance from urethra and from bladder floor. Thus, it possible to induce a true debulking of prostatic lobes with a subsequent reduction in volume over time with no bloch the urethral cavity and/or damage to urethral wall. The use of fine and multiple applicators with caliber less than 1 mm allows a relatively easy and safe transperineal approach and to tailor the number of heat sources and illuminations based on the shape and volume of the prostatic hyperplasia without harming adjacent structures. In addition, this laser treatment can be performed under conscious sedation and with local anesthesia during inpatient regimen of 2 days.
Some limitations of the present study have to be taken into account. First, this is the very first experience with a novel technique and is based on a very small series of patients. Moreover, as the primary endpoints of the study were feasibility and safety, only a short-time follow-up is provided. Then, the procedures were performed by a very experienced interventional radiologist, with more than 20 years of experience in US-guided thermal ablation. Thus, the result of this study is not easy to be generalized. Further studies on larger patient population and with longer follow-up, possibly comparing this novel technique with TURP, are needed to better define the role of the technique.
In conclusion, this study demonstrated that TPLA is feasible and safe in the treatment of patients with lower urinary symptoms due to BPH, with significant results after 3 months from the treatment.
References
Oelke M, Bachmann A, Descazeaud A, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64(1):118–40.
Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)—incidence, management, and prevention. Eur Urol. 2006;50(5):969–79 (discussion 80).
Rieken M, Bachmann A. Laser treatment of benign prostate enlargement—which laser for which prostate? Nat Rev Urol. 2014;11(3):142–52.
Zhang X, Shen P, He Q, et al. Different lasers in the treatment of benign prostatic hyperplasia: a network meta-analysis. Sci Rep. 2016;6:23503.
Stovsky MD, Griffiths RI, Duff SB. A clinical outcomes and cost analysis comparing photoselective vaporization of the prostate to alternative minimally invasive therapies and transurethral prostate resection for the treatment of benign prostatic hyperplasia. J Urol. 2006;176(4 Pt 1):1500–6.
Rosario DJ, Phillips JT, Chapple CR. Durability and cost-effectiveness of transurethral needle ablation of the prostate as an alternative to transurethral resection of the prostate when alpha-adrenergic antagonist therapy fails. J Urol. 2007;177(3):1047–51 discussion 51 .
Aoun F, Marcelis Q, Roumeguere T. Minimally invasive devices for treating lower urinary tract symptoms in benign prostate hyperplasia: technology update. Res Rep Urol. 2015;7:125–36.
Perera M, Roberts MJ, Doi SA, Bolton D. Prostatic urethral lift improves urinary symptoms and flow while preserving sexual function for men with benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol. 2015;67(4):704–13.
Jones P, Rai BP, Aboumarzouk OM, Somani BK. Prostatic urethral lift vs prostate arterial embolization: novel nonablative strategies in the management of lower urinary tract symptoms secondary to benign prostate hyperplasia. Urology. 2016;87:11–7.
Carnevale FC, Iscaife A, Yoshinaga EM, Moreira AM, Antunes AA, Srougi M. Transurethral resection of the prostate (TURP) versus original and PErFecTED prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovasc Intervent Radiol. 2016;39(1):44–52.
Dixon C, Cedano ER, Pacik D, et al. Efficacy and safety of rezum system water vapor treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2015;86(5):1042–7.
McVary KT, Gange SN, Gittelman MC, et al. Minimally nvasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016;195(5):1529–38.
Gilling P, Reuther R, Kahokehr A, Fraundorfer M. Aquablation—image-guided robot-assisted waterjet ablation of the prostate: initial clinical experience. BJU Int. 2016;117(6):923–9.
Roberts WW, Teofilovic D, Jahnke RC, Patri J, Risdahl JM, Bertolina JA. Histotripsy of the prostate using a commercial system in a canine model. J Urol. 2014;191(3):860–5.
Elhilali MM, Pommerville P, Yocum RC, Merchant R, Roehrborn CG, Denmeade SR. Prospective, randomized, double-blind, vehicle controlled, multicenter phase IIb clinical trial of the pore forming protein PRX302 for targeted treatment of symptomatic benign prostatic hyperplasia. J Urol. 2013;189(4):1421–6.
Pacella CM, Francica G, Di Lascio FM, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol. 2009;27(16):2615–21.
Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–30.
Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100(10):3903–10.
Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–1705.e4.
Kedia KR. Local anesthesia during interstitial laser coagulation of the prostate. Rev Urol. 2005;7(Suppl 9):S23–8.
Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–202.
Uchida T, Ohori M, Soh S, et al. Factors influencing morbidity in patients undergoing transurethral resection of the prostate. Urology. 1999;53(1):98–105.
Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5(2):209–16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Claudio Maurizio Pacella is consultant for Elesta s.r.l. All other authors have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Patelli, G., Ranieri, A., Paganelli, A. et al. Transperineal Laser Ablation for Percutaneous Treatment of Benign Prostatic Hyperplasia: A Feasibility Study. Cardiovasc Intervent Radiol 40, 1440–1446 (2017). https://doi.org/10.1007/s00270-017-1662-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1662-9