Abstract
Purpose
To use absolute pretreatment apparent diffusion coefficients (ADC) derived from diffusion-weighted MR imaging (DWI) to predict response to repetitive cTACE for unresectable liver metastases of colorectal carcinoma (CRLM) at 1 and 3 months after start of treatment.
Materials and Methods
Fifty-five metastases in 34 patients were examined with DWI prior to treatment and 1 month after initial cTACE. Treatment was performed in 4-week intervals. Response was evaluated at 1 and 3 months after start of therapy. Metastases showing a decrease of ≥30% in axial diameter were classified as responding lesions.
Results
One month after initial cTACE, seven lesions showed early response. There was no significant difference in absolute pretreatment ADC values between responding and non-responding lesions (p = 0.94). Three months after initial cTACE, 17 metastases showed response. There was a significant difference (p = 0.021) between absolute pretreatment ADC values of lesions showing response (median 1.08 × 10−3 mm2/s) and no response (median 1.30 × 10−3 mm2/s). Pretreatment ADC showed fair diagnostic value to predict response (AUC 0.7). Lesions showing response at 3 months also revealed a significant increase in ADC between measurements before treatment and at one month after initial cTACE (p < 0.001). Applying an increase in ADC of 12.17%, response at 3 months after initial cTACE could be predicted with a sensitivity and specificity of 77 and 74%, respectively (AUC 0.817). Furthermore, there was a strong and significant correlation (r = 0.651, p < 0.001) between percentage change in size after third cTACE and percentage change in ADC.
Conclusion
In patients with CRLM, ADC measurements are potential biomarkers for assessing response to cTACE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2012, colorectal cancer (CRC) was the third most common cancer among men and the second most common among women [1]. The liver is the most common site of metastases of CRC [2]. Transarterial chemoembolization (TACE) is a catheter-based regional therapy by interventional radiologists that is mainly used in cases with advanced hepatic involvement of CRC in a palliative situation, but has also been used in a neoadjuvant setting followed by surgical resection or percutaneous thermal ablation [3].
Assessment of response to TACE is usually performed by measuring tumor size on MR or CT imaging. In this context, identifying appropriate functional biomarkers is clinically important for the prediction of tumor response to TACE early and accurately, preferably before there is a morphological detectable change in size. Ultimately, such biomarkers may lead to change or cessation of ineffective therapy in non-responding patients.
One such quantitative biomarker may be the apparent diffusion coefficient (ADC), which is acquired by using diffusion-weighted MR imaging (DWI) [4]. The degree of diffusion is naturally limited by tissue properties, since cell density and organization as well as microcirculation highly influence the movements of water molecules. In comparison with extracellular diffusion, intracellular diffusion is limited by the cell membrane as a natural barrier. Diffusion is restricted in malignant tumors and abscesses, which show an increase in cell density, whereas necrotic tissues and cysts present with unimpeded diffusion. In DWI, diffusion is quantified by calculating the apparent diffusion coefficient [5, 6]. It has been shown that DWI can be used to characterize liver lesions when applied in abdominal imaging [7, 8]. Several studies have also demonstrated the potential utility of ADC as an indicator of response to TACE in patients with hepatocellular carcinoma (HCC) [9, 10] and liver metastases [11–13]. The purpose of our study was to assess the value of measuring ADC derived from DWI to evaluate tumor response to lipiodol-based TACE in patients with colorectal cancer liver metastases (CRLM).
Material and Methods
Patients
This retrospective study was approved by the local ethics committee with a waiver for written informed research consent. The patients’ clinical information was provided by medical records and by our institute’s database of patients undergoing interventional procedures. Using DWI, we retrospectively examined 55 unresectable liver metastases in 34 patients (mean 1.62 lesions per patient) with CRC that were treated between April 2010 and November 2015 with conventional lipiodol-based chemoembolization (cTACE). Patients’ characteristics are summarized in Table 1. All patients had the primary tumor surgically removed prior to interventional therapy. In total, 212 chemoembolizations were performed (mean 6.2 procedures per patient, range 3–16).
cTACE Technique
All TACE procedures were performed by an interventional radiologist with 20 years of experience. Following local anesthesia, the superficial femoral artery was catheterized using the Seldinger technique [14]. Under angiographic guidance, a 4-French catheter (Cobra, Terumo, Tokyo, Japan) was introduced into the celiac trunk and advanced into the hepatic artery and further into segmental arteries that supplied the targeted lesion. This was followed by the administration of a mixture of chemotherapeutics and embolic materials [15]. For exclusion criteria for cTACE, see Table 2.
At our institute, we use a combination of the following three chemotherapeutics in cTACE: mitomycin C (Medac 8 mg/m2, Germany), irinotecan (Campto 150 mg/m2, Pfizer Pharma, Germany) and cisplatin (Cisplatin Teva 35 mg/m2, Teva, Germany). This is followed by embolization with a maximum dose of 10 ml ethiodized oil (Lipiodol, Guerbet, France) and 200–450 mg of degradable starch microspheres (EmboCept 200 µm; PharmaCept, Germany) until stasis of blood flow in tumor-feeding arteries is observed under fluoroscopic control. This lipiodol-based chemoembolization is also referred to as conventional transarterial chemoembolization (cTACE) [16].
Imaging and Treatment Protocol
All patients received a minimum of three repetitive cTACE treatments in 4-week intervals. All imaging studies were obtained using a 1.5-Tesla MRI (Magnetom Espree, Siemens, Germany) scanner. Pretreatment imaging was performed with unenhanced and contrast-enhanced T1- and T2-weighted images. Plain MRI scans (T1-weighted spin-echo (SE) and T1-weighted gradient echo (GRE) sequences) were obtained before every subsequent procedure. Additionally, axial diffusion-weighted MR imaging was performed with a breath-hold technique with b-values of 50, 400 and 800 s/mm2 (repetition time = 950 ms, echo time = 82 ms, slice thickness = 6 mm, field of view = 380 ms and matrix size 91 × 192) prior to cTACE and at one month after initial cTACE. ADC values were extracted from ADC maps by manually drawing regions of interest around individual lesions. These ADC maps were automatically generated by the PACS workstation, which has been reported to be as accurate as a dedicated specialized workstation [17]. Percentage changes of ADC values between measurements were calculated. On the pretreatment images, a maximum of two target lesions per patient were identified that were suitable for repeated DWI measuring. As patients with a large number of metastases could potentially cause statistical bias, a maximum of two metastases per patient were evaluated. Only liver metastases larger than 1 cm in axial diameter were included. Whenever possible, the largest lesions were selected. Metastases that were located close to each other were excluded as confluence of lesions under therapy can limit accurate measurement of size [18]. Lesions in the left lobe of the liver, located close to blood vessels or showing artifacts in DWI or on ADC maps, were avoided for analysis as this can restrict accurate ADC measurements [19]. Of the identified target lesions, ADC values and maximum axial diameters were recorded. Axial diameter was measured on T1-weighted images. Response to treatment was evaluated on a lesion-by-lesion basis by recording percentage change in size at two points of our study: at one month after the beginning of cTACE and at one month after third cTACE (3 months after initial therapy). Evaluation of response at one month after third cTACE was chosen as the literature review suggests that metastases targeted by repetitive cTACE show maximum response following third session [20]. For the purpose of our study, lesions that showed a decrease of ≥30% in axial diameter were categorized as responding lesions, while all other metastases were classified as non-responding lesions.
Statistical Analyses
All statistical analyses were performed using the “Statistical Package for the Social Science statistics” version 23.0 (SPSS Statistics, IBM, USA). P values <0.05 were considered statistically significant. The Mann–WhitneyU Test was used to compare pretreatment ADC values between lesions showing response and no response following cTACE therapy. ADC values before and after treatment were compared using the Wilcoxon signed-rank test. To evaluate percentage changes of ADC values between responding and non-responding lesions, independent two-sample t test was applied.
Spearman rank and Pearson correlation coefficients were used to assess the correlation between size and ADC values. Results of correlation analysis were evaluated according to Evans [21]. Receiver operating characteristic (ROC) curve analyses were used to determine whether pretreatment ADC values or change of ADC values can predict response to cTACE at 1 and 3 months after initial cTACE.
Results
Pretreatment Values
Fifty-five liver lesions in 34 patients were identified to be suitable for therapy monitoring with DWI. Pretreatment median size of evaluated lesions was 34.1 mm (range 12.6–156.8, mean 41.11, SD 25.57). Median ADC prior to therapy of all examined lesions was 1.16 × 10−3 mm2/s (range 0.65–3.17, mean 1.36, SD 0.51). There was a poor correlation between pretreatment size and pretreatment ADC values (r = 0.141, p = 0.303).
Results After First cTACE
One month after initial cTACE, response and ADC values were evaluated. Forty-eight lesions showed no response, while seven lesions showed early response. Mean percentage change in size for all lesions showed a decrease of 5.33% (SD 21.47, median decrease 1.64). Non-responding lesions showed a mean increase in diameter of 0.16% (SD 16.76, median increase 0.18). Responding lesions showed a mean decrease in diameter of 42.96% (SD 7.44, median decrease 40.86). There was no significant difference in absolute pretreatment ADC values of lesions exhibiting response (median 1.13 × 10−3 mm2/s, mean 1.21, SD 0.16) and no response (median 1.17 × 10−3 mm2/s, mean 1.38, SD 0.54) at one month after first cTACE (p = 0.94). ROC analysis showed an area under the curve (AUC) of 0.491 for using pretreatment ADC to predict response at one month, which indicated no predictive value. Correlation between absolute pretreatment ADC values and percentage change in metastases size at one month following cTACE was also poor (r = 0.195, p = 0.153). In addition to the evaluation of treatment response, ADC values of liver lesions were also obtained at one month following first cTACE. Responding lesions showed a median ADC of 1.53 × 10−3 mm2/s (mean 1.61, SD 0.18) and non-responding lesions a median ADC of 1.31 × 10−3 mm2/s (mean 1.43, SD 0.50). These absolute values showed no statistically significant difference (p = 0.177). Percentage changes in ADC values were also calculated. In non-responding lesions, a mean increase of 6.39% (SD 22.29, median increase 0.51) was observed, whereas responding metastases showed a mean increase of 26.59% (SD 16.44, median increase 16.62) in ADC values. There was a statistically significant difference between these two groups (p = 0.017). Additionally, there was a moderate but significant correlation (r = 0.556, p < 0.001) between percentage change in size and percentage change in ADC values, with lesions presenting with a greater increase in ADC values showing better response to cTACE.
Results After Third cTACE
One month after third cTACE (3 months after initial cTACE), response was again evaluated. Thirty-eight lesions showed no response, while 17 lesions showed response. Mean percentage change in size for all lesions showed a decrease of 4.05% (SD 42.68, median decrease 7.62). Non-responding lesions showed a mean increase in diameter of 16.73% (SD 33.58, median increase 11.49). Responding lesions showed a mean decrease in diameter of 50.51% (SD 14.85, median decrease 48.62). There was a statistically significant difference between absolute pretreatment ADC values of responding (median 1.08 × 10−3 mm2/s, mean 1.13, SD 0.20) and non-responding (median 1.30 × 10−3 mm2/s, mean 1.46, SD 0.57) lesions at 3 months after initial cTACE (p = 0.021). With an AUC of 0.7 in the ROC analysis, pretreatment ADC was identified as a fair predictor of response at 3-month follow-up.
Percentage changes between pretreatment values and ADC values after first cTACE were also compared for lesions exhibiting response at 3 months, which showed a statistically significant difference (p < 0.001) (Fig. 1).
Absolute pretreatment apparent diffusion coefficients (ADC) and ADC values at one month following initial lipiodol-based transarterial chemoembolization (cTACE) of liver metastases showing response (n = 17) and no response (n = 38) at 3 months after initial cTACE. Difference of pretreatment ADC values of responding and non-responding lesions was statistically significant (p = 0.021). Increase in ADC in responding lesions was also significant (p < 0.001). ADC measured in 10−3 mm2/s
Percentage change in ADC values showed an AUC of 0.817, indicating a good predictive value for response at 3 months. A cutoff value of percentage change was chosen that yielded maximal sensitivity and specificity. An increase of 12.17% between pretreatment ADC values and ADC values at one month after first cTACE had 77% sensitivity and 74% specificity to predict response following three sessions of cTACE (Fig. 2).
Receiver operating characteristic (ROC) analysis for predicting response in lesions at 3 months after initial cTACE with percentage change in ADC values. An area under the curve (AUC) of 0.817 indicated a good predictive value. A threshold of an increase of 12.17% in change of ADC values yielded 77% sensitivity and 74% specificity to predict response in targeted lesions
Correlation between pretreatment ADC values and percentage change in metastases size at 3 months was weak but significant (r = 0.281, p = 0.038). There was a strong and highly significant correlation (r = 0.651, p < 0.001) between percentage change in size at 3 months following initial cTACE and percentage change in ADC values (Fig. 3).
Percentage change of size in liver metastases 3 months after initial cTACE and percentage change in ADC values. Line through y-axis (−30) separates responding from non-responding lesions. Line through 12.17 on x-axis indicates threshold to predict response with 77% sensitivity and 74% specificity. Graph also shows linear regression between percentage change in size and percentage change in ADC (percentage change of size = 6.99 − (1.23 × percentage change of ADC); r = 0.651, p < 0.001
Discussion
This study was carried out to investigate the diagnostic value of ADC measurements in order to predict and monitor response of CRLM to cTACE treatment. In this study, we further defined a predictive relationship between pretreatment ADC and CRLM response to cTACE as well as the mirroring of CRLM changes throughout treatment in corresponding ADC changes. Thus, our data demonstrate that ADC values can be used for monitoring the response of colorectal carcinoma liver metastases to cTACE.
Recently, several studies have been conducted addressing the role of DWI in predicting response to systemic and locoregional chemotherapy in patients with different tumor entities. Liver tumors usually present with more restricted diffusion than physiological liver parenchyma [8, 22]. With regard to this observation, it has been investigated whether tumors with more restricted diffusion (lower ADC values) prior to treatment exhibit better response to interventional therapies than others. Kokabi et al. [23] demonstrated that response at 1 and 3 months after drug-eluting bead chemoembolization of unresectable hepatocellular carcinoma could be predicted with high sensitivity and specificity for lesions with pretreatment ADC values of <0.83 × 10−3 mm2/s. The value of pretreatment DWI to predict response in CRLM has also been examined for systemic chemotherapy, indicating that lower pretreatment ADC values are associated with a better response [4, 24]. A rationale for this observation is that high ADC values indicate extended necrosis with consecutive poor perfusion, making such lesions less susceptible to chemotherapy [23, 25, 26]. To the best of our knowledge, so far there have been no studies assessing DWI as a tool to evaluate tumor response in patients with CRLM undergoing cTACE. In our study, pretreatment ADC values could not be identified as a reliable predictor of response at 3 months (AUC 0.7) (Fig. 4).
Buijs et al. [13] observed that there is a significant increase in ADC values of liver metastases from breast cancer targeted by TACE. Similar findings were reported in different hepatic metastases treated with systemic chemotherapy [24].
Several studies showed an association between the increase in ADC values and the degree of necrosis in histopathological analysis of HCC following therapy with TACE [9, 27]. Schmeel et al. [12] evaluated the change of ADC values of colorectal cancer metastases after selective internal radiation therapy (SIRT). With a sensitivity of 94% and a specificity of 82%, an increase of ≥22% of ADC allowed the identification of patients with above-average overall response. In our study, lesions exhibiting response at 3 months after initial cTACE showed a statistically significant increase in ADC values between pretreatment measurements and measurements at one month after initial chemoembolization (p < 0.001). The sensitivity and specificity of predicting response at 3 months after initial cTACE were 77 and 74%, respectively (AUC 0.817), if lesions showed an increase in ADC of ≥12.17% at one month following first cTACE (Fig. 5). Percentage change in size at 3 months following initial cTACE and percentage change in ADC values also showed a strong and significant correlation (r = 0.651, p < 0.001).
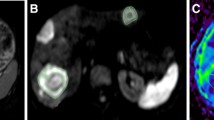
Metastases in liver segment IV showing response at 3 months after initial cTACE. The lesion demonstrated with a baseline ADC value of 1.02 × 10−3mm2/s. Following first cTACE, the ADC increased by 33.82%. Diffusion-weighted image was obtained by using a b-value of 50 s/mm2. Note renal cyst posterior to liver metastases presenting with a high ADC value of 2.99 × 10−3 mm2/s. ADC value of cyst remained unchanged during treatment with cTACE
Despite DWI’s promising potential in predicting response to systemic and locoregional chemotherapy in patients treated for a variety of tumor entities, there are still several issues that need to be addressed. In a study conducted on hepatic metastases treated with systemic chemotherapy, Deckers et al. [18] reported that ADC values showed a great variability between repetitive measurements. The authors also showed that the average changes in ADC values of responding lesions were of smaller magnitude compared with the measurement error of liver DWI, making ADC values an unreliable predictor of response. Calculating a normalized ADC by dividing the ADC of lesions through the ADC of normal liver parenchyma also did not minimize the variability between measurements. Obviously, reproducibility of ADC measurements of liver lesions has to be ensured in order to become a biomarker of treatment response. Additionally, comparability of studies examining DWI is somewhat lacking, as ADC values underlie variability depending on used MRI scanner and software. Furthermore, no accepted standards for measurements and analysis of ADC measurements have been established, and diffusion-weighted MR imaging is not broadly available routinely in many cancer centers [19, 28]. Our study had limitations that should be considered. We realize that choosing up to two metastases of the same patient potentially constitutes a bias, as related metastases potentially react similar to treatment compared to lesions in a different patient. In future studies, liver metastases that are not subject to cTACE in the same patients could be monitored for changes in ADC values in order to form an internal control group. A major limitation of our study was its retrospective design; prospective studies should try to confirm and further evaluate our promising results.
Also, such studies should try to incorporate histological analysis of treated liver metastases in order to further evaluate the association of necrosis and ADC values and its changes during cTACE therapy. In conclusion, we demonstrated the potential use of ADC measurements derived from DWI as a biomarker for assessing response to cTACE in patients with CRLM.
References
McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer, WHO Press, 2015. Adv Nutr. 2016;7(2):418–9. doi:10.3945/an.116.012211.
Jonker DJ, Maroun JA, Kocha W. Survival benefit of chemotherapy in metastatic colorectal cancer: a meta-analysis of randomized controlled trials. Br J Cancer. 2000;82(11):1789–94. doi:10.1054/bjoc.1999.1254.
Gruber-Rouh T, Naguib NN, Eichler K, Ackermann H, Zangos S, Trojan J, et al. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer. 2014;134(5):1225–31. doi:10.1002/ijc.28443.
Koh D-M, Scurr E, Collins D, Kanber B, Norman A, Leach MO, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. Am J Roentgenol. 2007;188(4):1001–8. doi:10.2214/AJR.06.0601.
Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45(3):169–84.
Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16(13):1567–76.
Koh DM, Collins DJ, Wallace T, Chau I, Riddell AM. Combining diffusion-weighted MRI with Gd-EOB-DTPA-enhanced MRI improves the detection of colorectal liver metastases. Br J Radiol. 1015;2012(85):980–9. doi:10.1259/bjr/91771639.
Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiogr A Rev Publ Radiol Soc North Am Inc. 2009;29(6):1797–810. doi:10.1148/rg.296095521.
Chapiro J, Wood LD, Lin M, Duran R, Cornish T, Lesage D, et al. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273(3):746–58. doi:10.1148/radiol.14140033.
Chung JC, Naik NK, Lewandowski RJ, Deng J, Mulcahy MF, Kulik LM, et al. Diffusion-weighted magnetic resonance imaging to predict response of hepatocellular carcinoma to chemoembolization. World J Gastroenterol. 2010;16(25):3161–7.
Venturini M, Pilla L, Agostini G, Cappio S, Losio C, Orsi M, et al. Transarterial chemoembolization with drug-eluting beads preloaded with irinotecan as a first-line approach in uveal melanoma liver metastases: tumor response and predictive value of diffusion-weighted MR imaging in five patients. J Vasc Interv Radiol. 2012;23(7):937–41. doi:10.1016/j.jvir.2012.04.027.
Schmeel FC, Simon B, Sabet A, Luetkens JA, Traber F, Schmeel LC, et al. Diffusion-weighted magnetic resonance imaging predicts survival in patients with liver-predominant metastatic colorectal cancer shortly after selective internal radiation therapy. Eur Radiol. 2016;. doi:10.1007/s00330-016-4430-3.
Buijs M, Kamel IR, Vossen JA, Georgiades CS, Hong K, Geschwind JF. Assessment of metastatic breast cancer response to chemoembolization with contrast agent enhanced and diffusion-weighted MR imaging. J Vasc Interv Radiol. 2007;18(8):957–63. doi:10.1016/j.jvir.2007.04.025.
Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol. 1953;39(5):368–76. doi:10.3109/00016925309136722.
Vogl TJ, Muller PK, Mack MG, Straub R, Engelmann K, Neuhaus P. Liver metastases: interventional therapeutic techniques and results, state of the art. Eur Radiol. 1999;9(4):675–84. doi:10.1007/s003300050732.
Gruber-Rouh T, Marko C, Thalhammer A, Nour-Eldin NE, Langenbach M, Beeres M, et al. Current strategies in interventional oncology of colorectal liver metastases. Br J Radiol. 2016;89:20151060. doi:10.1259/bjr.20151060.
El Kady RM, Choudhary AK, Tappouni R. Accuracy of apparent diffusion coefficient value measurement on pacs workstation: a comparative analysis. Am J Roentgenol. 2011;196(3):W280–4. doi:10.2214/AJR.10.4706.
Deckers F, De Foer B, Van Mieghem F, Botelberge T, Weytjens R, Padhani A, et al. Apparent diffusion coefficient measurements as very early predictive markers of response to chemotherapy in hepatic metastasis: a preliminary investigation of reproducibility and diagnostic value. J Magn Reson Imaging. 2014;40(2):448–56. doi:10.1002/jmri.24359.
Taouli B, Koh D-M. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254(1):47–66. doi:10.1148/radiol.09090021.
Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250(1):281–9. doi:10.1148/radiol.2501080295.
Evans JD. Straightforward statistics for the behavioral sciences. Pacific Grove: Brooks/Cole Publ. Co: An International Thomson Publ. Co; 1996.
Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI–a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5(4):220–33. doi:10.1038/ncponc1073.
Kokabi N, Ludwig JM, Camacho JC, Xing M, Mittal PK, Kim HS. Baseline and early MR apparent diffusion coefficient quantification as a predictor of response of unresectable hepatocellular carcinoma to doxorubicin drug-eluting bead chemoembolization. J Vasc Interv Radiol. 2015;26(12):1777–86. doi:10.1016/j.jvir.2015.08.023.
Cui Y, Zhang X-P, Sun Y-S, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248(3):894–900. doi:10.1148/radiol.2483071407.
Thoeny HC, De Keyzer F, Chen F, Vandecaveye V, Verbeken EK, Ahmed B, et al. Diffusion-weighted magnetic resonance imaging allows noninvasive in vivo monitoring of the effects of combretastatin a-4 phosphate after repeated administration. Neoplasia (New York, NY). 2005;7(8):779–87.
Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9(Suppl 5):31–40. doi:10.1634/theoncologist.9-90005-31.
Mannelli L, Kim S, Hajdu CH, Babb JS, Taouli B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: prediction and assessment of response to transarterial chemoembolization. Prelim Exp Eur J Radiol. 2013;82(4):577–82. doi:10.1016/j.ejrad.2012.11.026.
Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia (New York, NY). 2009;11(2):102–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lahrsow, M., Albrecht, M.H., Bickford, M.W. et al. Predicting Treatment Response of Colorectal Cancer Liver Metastases to Conventional Lipiodol-Based Transarterial Chemoembolization Using Diffusion-Weighted MR Imaging: Value of Pretreatment Apparent Diffusion Coefficients (ADC) and ADC Changes Under Therapy. Cardiovasc Intervent Radiol 40, 852–859 (2017). https://doi.org/10.1007/s00270-017-1634-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1634-0