Abstract
Purpose
To evaluate the technical feasibility and clinical efficacy of placement of a newly designed Y-shaped branched covered stent for palliative treatment of malignant hilar biliary obstruction.
Methods
From June 2011 to September 2014, 34 consecutive patients with malignant hilar biliary obstruction underwent percutaneous placement of a Y-shaped branched covered stent for palliative treatment. Technical and clinical success, complications, cumulative patient survival, and stent patency were evaluated.
Results
Stent placement was technically successful in all patients. All patients showed adequate biliary drainage on the follow-up cholangiogram. Mean serum bilirubin level (10.9 mg/dl) decreased significantly 1 week (5.7 mg/dl) and 1 month (2.6 mg/dl) after stent placement (p < 0.01). Complications associated with the procedure included hemobilia (n = 3) and biloma (n = 1). During the mean follow-up period of 225 (range 12–820) days, nine patients (26.5 %) developed stent occlusion caused by tumor overgrowth (n = 8) and sludge (n = 1). Two of them underwent coaxial placement of a second stent with good results. The median survival time was 281 days and median primary stent patency was 337 days. There were no significant differences in the patient survival and stent patency rates in relation to age, sex, or Bismuth type.
Conclusion
Percutaneous placement of the Y-shaped branched covered stent seems to be technically feasible and clinically effective for palliative treatment of malignant hilar biliary obstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant hilar biliary obstruction results in jaundice, pruritus, pain, cholangitis, and often decreasing the quality of life. It has extremely poor prognosis with reported a 5-year survival rate less than 10 % [1, 2]. Although surgical resection offers best possibility of prolonged survival, overall resectability rates for hepatic hilar malignancies are reported to be approximately 10–20 % [2]; therefore, treatment in most patients is mainly palliative rather than curative.
Percutaneous or endoscopic metallic stent insertion has recently become a widely accepted procedure for palliative treatment of malignant hilar biliary obstruction. Many authors have described new devices and various techniques for bilateral bare metallic stent placement across the hilar malignancy, including T-configured [3–6], Y-configured [5–8], and crisscross-configured dual stent placement [5, 9]. Moreover, successful use of covered stent was also reported in patients with malignant hilar biliary obstruction to provide durable stent patency without tumor ingrowth [10, 11]. When the stent is obstructed by tumor ingrowth or sludge formation, however, management of stent occlusion by means of re-stenting can be technically difficult; in most hilar biliary obstruction, bilateral stents are placed using the stent-in-stent fashion, and the wires of the previously inserted stent have a potential risk of preventing the additional stent insertion through the contralateral bile duct [7, 12].
Recently, a new design of Y-shaped branched covered stent was devised to improve the stent patency rate and to allow feasible management of stent occlusion [13]. The purpose of this study is to evaluate the technical feasibility and clinical efficacy of using this stent for palliative treatment of 34 patients with malignant hilar biliary obstruction.
Materials and Methods
Patients
The institutional review board of our university approved this retrospective study, and written informed consent was waived from each patient. From June 2011 to September 2014, 34 consecutive patients with malignant hilar biliary obstruction underwent placement of an Y-shaped branched covered stent. Their demographic, clinical, and laboratory data were collected from their medical records, histopathologic reports, and diagnostic imaging reports of each patient. The radiologic and clinical follow-up data were recorded in all patients. There were 23 male and 11 female, ranging in age from 38 to 92 (mean 71) years. The causes of hilar obstruction were cholangiocarcinoma in 31 patients, and metastatic carcinoma in three. The diagnosis of malignant disease was confirmed by pathologic findings from percutaneous transluminal forceps biopsy (n = 19) or surgical specimen (n = 1), or radiologic follow-up with evidence of increase in extent of the lesion (n = 14). The types of hilar obstruction based on the Bismuth classification were type I (n = 2), type II (n = 20), type IIIa (n = 9), type IIIb (n = 1), and type IV (n = 2). All patients were considered inoperable because of their tumor extension or comorbid conditions (Table 1).
Devices
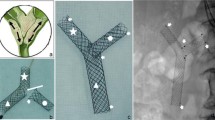
The Y-shaped branched covered stent (EGIS Biliary KEY stent; S&G Bio, Seoul, Korea) consisted of two components of silicone covered nitinol stent (Fig. 1): a main piece of the stent and a contralateral limb stent, which are introduced separately and mated in place. The main piece of the stent was comprised a common bile duct (CBD) section (uncovered stent), one short limb (covered stent), and one long limb (covered stent). The CBD section of the stent was 10 mm in diameter and 10 mm long. The short limb was 7 mm in diameter and 10 mm long. The long limb was 7 mm in diameter and 40–70 mm long (7 mm proximal bare end). Radiopaque markers made of gold were attached at each ends of the stent and covered end of the long limb of the stent. Two radiopaque gold markers were also attached at the lateral side of the short limb to serve as a reference to the direction of the short limb during the deployment of the main piece of the stent. The contralateral limb stent was 7 mm in diameter and 50–80 mm long (3.5 mm distal bare end and 7 mm proximal bare end). Radiopaque gold markers were attached at both bare and covered ends. Y-shape of the stent was achieved by docking short limb of the main piece of the stent with the contralateral limb stent to form a continuous channel (Fig. 1B). Both the main piece of the stent and contralateral limb stent were delivered through an 8-F introducer sheath made of Teflon.
A Photograph of a Y-shaped branched covered stent, which has two components: a main piece of the stent (arrow) and a contralateral limb stent (black arrowhead). The main piece is comprised a CBD section (C), a long limb (L), and a short limb (S). White arrowheads indicate two lateral radiopaque markers. B Y-shape of the stent is achieved by docking short limb of the main piece of the stent with the contralateral limb stent
Technique
Before the procedure, computed tomography (CT) or magnetic resonance cholangiopancreatography was obtained for the evaluation of the tumor extent, anatomy of the biliary tree and the most appropriate approach to both lobes of the liver. Broad-spectrum antibiotics were routinely administered for both the drainage and stent placement procedure. For conscious sedation, pethidine hydrochloride (Demerol, Keukdong Pharmaceuticals, Seoul, Korea) was administered intravenously prior to the procedure. Percutaneous transhepatic biliary drainage (PTBD) was performed to relieve jaundice and cholangitis using the standard technique under the ultrasound and fluoroscopic guidance. A drainage catheter (Cook, Inc, Bloomington, Indiana) was inserted for unilobar drainage, or across the hilar tumor to the contralateral lobe for bilobar drainage, if possible. In patients with persistent cholangitis despite an initial unilobar drainage, contralateral PTBD was performed 2–3 days after initial procedure.
All stents were decided to be placed after PTBD, and were placed 3–7 days after the drainage procedure. The stent placement was performed via bilateral approach in all patients and the technique is illustrated in Figs. 2 and 3. A new access to the contralateral bile duct was performed in patients with one PTBD tract. An 8-F introducer sheath was inserted through each PTBD tract. After hilar strictures were negotiated with a 0.035-inch, 150-cm-long hydrophilic guide wire (Terumo, Tokyo, Japan) from each intrahepatic duct (IHD) through the CBD into the duodenum, the strictures were dilated using a 7-mm balloon catheter (Synergy; Boston Scientific, Galway, Ireland) before stent placement. The main piece of the stent was first introduced over the guide wire into the CBD usually through the left PTBD tract. The proximal end of the main piece of stent was located in the ipsilateral IHD beyond the boundary of the hilar tumor. Then, the lateral radiopaque markers at the distal portion of the stent was positioned correctly toward the ipsilateral side under fluoroscopy as the lateral radiopaque markers indicated the direction of the short limb of the main piece in the CBD. Only short limb part was then deployed in order to easily insert the contralateral limb stent coaxially into the short limb stent. To insert a contralateral limb stent, a 5-F angled tip catheter (Kumpe; Cook, Inc) was inserted over the guide wire through the contralateral PTBD tract. The guide wire was withdrawn carefully together with the catheter up to just the proximal portion of the deployed short limb and then advanced coaxially through the open end of short limb into the duodenum. A contralateral limb stent was then introduced over the guide wire and was advanced until the distal portion of the contralateral stent completely overlapped the short limb of the main piece. After confirmation of its proximal end located in IHD beyond the boundary of the hilar tumor and its distal end located within the lumen of the short limb stent, the contralateral limb stent was deployed. Finally, the long limb of the main piece of the stent was fully deployed. An 8.5-F drainage catheter was placed with its tip in the CBD. To verify the position and patency of the stent, cholangiography was performed through the catheter 3–6 days after the procedure. After patency of the expanded stents was confirmed by the cholangiogram, the drainage catheter was then removed. Information concerning current status or death was obtained for all patients from medical records or by telephone contact.
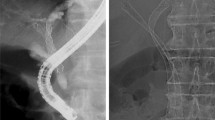
Hilar cholangiocarcinoma (Bismuth type III) in a 92-year-old woman. A Spot radiograph obtained during the procedure shows a malignant hilar obstruction. After negotiation of each stricture with the use of a guide wire and a 5-F catheter, the main piece of the stent was inserted over the guide wire into the CBD through the left PTBD tract. Notice the lateral radiopaque markers (arrowheads) facing toward left side in the CBD. Arrow indicates radiopaque markers at the end of the short limb. B Deployment of the main piece of the stent was proceeded until only the short limb was fully deployed. C The guide wire was advanced through open end of the short limb into the duodenum, and the contralateral limb stent (arrows) was coaxially inserted into the short limb. D After verification of the distal end of the contralateral limb stent completely overlapping the short limb of the main piece, the stent was fully deployed. E Cholangiogram after full deployment of the rest of the main piece, shows good contrast flow through the stent. F Cholangiogram obtained 3 days after the procedure shows good stent position and bilateral internal drainage through the stent
Definition
The technical and clinical success rate, complications, and cumulative patient survival and stent patency were evaluated. Technical success was defined as deployment of the stent across the hilar stricture with adequate radiologic positioning and good contrast flow through the stent. Clinical success was defined as successful removal of drainage catheters and decrease in serum bilirubin level to less than 75 % of the pretreatment value on 1 month follow-up study. Complications of the procedures were classified into major and minor categories according to the guidelines of the Society of Interventional Radiology Standards of Practice Committee [14]. The stent was considered to be occluded when the patient had recurrent symptoms of biliary obstruction including jaundice and cholangitis with increase in serum bilirubin levels (>3 mg/dl) and bile duct dilatation on the imaging study. The stent patency period was defined as the interval between initial stent placement and recurrence of symptoms because of stent occlusion. If obstruction was not evident during a patient’s life, the patency period was considered equal to the survival period but censored. The patient survival period was defined as the interval between initial stent placement and patient’s death.
Statistical Analysis
Paired-sample Student t test was used to compared the serum bilirubin levels before and after the stent placement. Cumulative patient survival and stent patency were calculated according to the Kaplan–Meier methods. Log-rank test was used to compare patient survival and stent patency according to age, sex, or Bismuth type of obstruction. Statistical significance was assumed at p value less than 0.05. Statistical procedures were performed using the statistical package of SPSS (version 19.0, SPSS, Chicago, Illinois).
Results
On initial PTBD, an 8.5-F drainage catheter (Cook, Inc) was inserted across the hilar tumor to the contralateral lobe for bilobar drainage in 25 patients and in the ipsilateral lobe for unilobar drainage in nine. Contralateral PTBD was performed in five of nine patients with initial unilobar drainage, 2–3 days after initial procedure because of persistent cholangitis. Therefore, 29 patients underwent an additional contralateral PTBD during the stent placement procedure.
Stent placement was technically successful in all patients (Fig. 3). The main piece of the stent was inserted through the left PTBD tract in 30 patients and through the right tract in three. In the remaining one patient with recurrent cholangiocarcinoma after previous left lobectomy, stent placement was successfully performed through two right accesses to drain both the right anterior and posterior duct. Procedure-related minor complications occurred in four patients (11.8 %), including hemobilia (n = 3) and biloma (n = 1). All cases with hemobilia were transient and resolved without transfusion within 2 days after stent placement. One case with biloma was successfully treated by percutaneous catheter drainage. There were no major complications associated with the procedure.
Follow-up cholangiograms obtained 3–6 days after stent placement showed adequate biliary drainage with decompression of the treated biliary ducts in all patients (Fig. 3). The drainage catheters were removed from all patients after confirmation of the good contrast flow through the stent into the duodenum. In 20 patients, one to three branching segmental ducts blocked by the covered portion of the stent were not opacified by contrast media on follow-up cholangiogram. No segmental duct blockage developed in the remaining 14 patients with Bismuth type II.
Initial serum bilirubin level ranged from 1.4 to 30.1 mg/dl (mean 10.9 mg/dl). Mean serum bilirubin level decreased significantly 1 week (range 0.58–21.8 mg/dl, mean 5.7 mg/dl) and 1 month (range 0.5–10.9 mg/dl, mean 2.6 mg/dl), respectively, after stent placement (p < 0.01). Patients both with and without segmental duct blockage on follow-up cholangiogram after stent placement, showed a significant decrease in the serum bilirubin level (Table 2).
During the mean follow-up period of 225 (range 12–820) days, thirty of 34 patients died. Two patients (5.9 %) died during the first month after the procedure because of rapid progression of the underlying malignancy. Four patients were lost to follow-up at 290, 313, 335, and 517 days, respectively, after stent placement. Until that time, they were still free of symptoms. According to the Kaplan–Meier analysis, the median patient survival time was 281 days (95 % CI 182–380, mean 276 days). The 3, 6, 9, and 12 months survival rates were 79.4, 57.8, 50.6, and 23.5 %, respectively (Fig. 4).
During the follow-up, nine patients (26.5 %) developed recurrent symptoms of obstruction 64–377 (mean 204) days after the procedure, caused by stent occlusion due to tumor overgrowth (n = 8) and sludge formation (n = 1). Seven patients were treated by PTBD; unilobar (n = 3) or bilobar (n = 2) drainage with one catheter was possible in five patients, and the remaining two required bilobar drainage with two catheters. One patient was treated by endoscopic insertion of unilateral plastic stent. Remaining one refused further treatment and died 42 days later. In two patients who underwent PTBD due to tumor overgrowth, a second limb stent (7 mm in diameter) was placed coaxially into the original limb with good results. Once the stent occlusion was negotiated using a hydrophilic guide wire (Terumo) and a 5-F angled tip catheter (Kumpe; Cook, Inc), the second stent was able to be placed without difficulty. However, in one patient with stent occlusion due to tumor overgrowth, second stent placement was tried but failed due to failure in negotiation of the guide wire through the stent occlusion. According to the Kaplan–Meier analysis, the median stent patency time was 337 days (95 % CI 269–405, mean 339 days). The 3, 6, 9, and 12 months patency rates were 93, 88.3, 70.5, and 33.4 %, respectively (Fig. 5). There was no significant difference in the patient survival and stent patency rates in relation to age, sex, or Bismuth type (p = 0.813).
Discussion
Palliation with percutaneous metallic stent insertion has been the best option available for the treatment of patients with unresectable malignant hilar biliary obstruction, given the poor prognosis of these patients. Although the extent of liver drainage remains unclear in cases of malignant hilar obstruction, bilateral biliary drainage has become generally accepted in recent studies [5, 8, 10, 11, 15]. New devices and various techniques for bilateral metallic stent placement across the hilar malignancy have been reported, including T-configured, Y-configured, and crisscross-configured dual stent placement [3–11].
There are two techniques of deployment for bilateral metallic stent placement for advanced hilar malignancies: stent-in-stent deployment and side-by-side deployment. In stent-in-stent deployment, a second stent is placed through the mesh of the first stent from a single transhepatic access, which is the main advantage of this technique. However, the wires of the second stent were partially left inside the first stent at the confluence [4], and may impair the contralateral bile inflow in the area of stent overlap, leading to sludge formation [12]. Naitoh et al. [16] reported significantly worse stent patency for stent-in-stent deployment than for side-by-side deployment in their clinical comparison study between two techniques. Furthermore, the wires of the preexisting stent have a potential risk of preventing the additional stent insertion through the contralateral bile duct, when the stent occlusion occurred [7, 12]. On the other hand, side-by-side deployment is bilateral metallic stenting with simultaneous insertion of two parallel stents through separate transhepatic approaches. Side-by-side deployment has a double space at hilar region, thus allowing more effective bile drainage and more durable stent patency [16]. However, the parallel arrangement of two stents in this technique may prevent full expansion at and below the hepatic confluence, which may result in stent elongation or partial collapse of one or both stents [4, 11]. In addition, this technique may be associated with a high rate of late complications, particularly cholecystitis, because excessive expansion of the bile duct by the two parallel stents causes obstruction of the cystic duct [8, 16].
The Y-shaped branched covered stent used in the present study, adopted the design of side-by-side deployment for effective bile drainage. In order to prevent excessive expansion of the common duct, smaller-diameter limb stent (7 mm) was used. The CBD section (uncovered stent, 10 mm) of the main piece of the stent further prevent distension of the CBD. In our study, placement of the Y-shaped stent was safe and feasible with technical success in all patients. No major complication was associated with the procedure. In addition, cholecystitis did not occur in any patients. The technique of the stent placement is relatively simple. Two guide wires are separately inserted through the CBD into the duodenum via bilateral approach. After adequate placement of the main piece of the stent, short limb was selected with the guide wire from contralateral PTBD tract and then the contralateral limb stent was inserted over the guide wire coaxially into the short limb of the main piece. In most of our patients, the main piece of the stent was inserted through the left PTBD tract because the left main duct is anatomically constant without significant variations except drainage of the right posterior bile [17], so the measurement from the CBD to the left main IHD followed by deploying the main body from the left side is easy and simple. It is also easy to negotiate the guide wire and then to insert the contralateral limb stent into the short limb of the main piece of the stent from the right side. There is one technical step which is quite different from the usual stenting procedure [13]. During the deployment of the main piece of the stent, one should be cautious to deploy only short limb and thus not to deploy the long limb before the insertion of the contralateral limb stent. If the long limb of the main piece is deployed completely, it may interrupt the negotiation of the guide wire and the following insertion of the contralateral limb stent passing through the hilar obstructive lesion into the short limb of the main piece of the stent. In addition, short limb should be positioned toward the ipsilateral side within the CBD in order to be easily selected with the guide wire from contralateral approach. Accurate direction of the short limb within the CBD is achieved by correct positioning of the lateral radiopaque marker of the stent toward the ipsilateral side under fluoroscopy.
A stent covering is proved to provide long-term patency of the stent because it can prevent tumor ingrowth [10, 11, 15]. In one prospective randomized study comparing covered and uncovered stents, the authors reported that covered stent was significantly superior to uncovered stent for the treatment of patients with distal malignant biliary obstruction. In our study, the median patient survival and stent patency time were 281 and 337 days, respectively. These figures compare quite favorably with the results of uncovered stent placement for malignant hilar biliary obstructions, ranging from 193.6 to 299 days of median survival time, and 140 to 191.8 days of median stent patency [4, 5, 7, 8]. On the other hand, a prospective study of covered stent placement for advanced hilar malignancies obtained results comparable to ours, i.e., the median patient survival time of 218 days and the median stent patency time of 375 days [11]. Various kinds of covering materials, such as polyurethane, e-PTFE, and silicone, have been currently used to cover the biliary metallic stents [10, 11, 18–22]. In our study, a silicone was used as the covering material because it is biodurable and provides a larger lumen for bile flow by means of alleviating biofilm and sludge formation [23, 24].
Despite the advantage of preventing tumor ingrowth, use of the covered stent can be plagued by stent migration [20–22, 25]. Various methods of preventing stent migration have been described [10, 11, 26]. In our study, no stent migration occurred, and we assume that our partially covered stent design with both bare ends (CBD section and long limb of the main piece, and contralateral limb) may resist migration of the stent. Another possible limitation of using covered stent for treating advanced hilar malignancies is a risk of occlusion of branching duct. Other researchers, however, have reported a significant decrease in diameter of the segmental ducts blocked by the covered portion of the stent on follow-up abdominal CT scans [10, 11]. Moreover, segmental ductal blockage by the covered stents did not cause a significant increase in serum bilirubin level or cholangitis [11]. They surmised that ductal blockage was not complete even when the stent fully expanded, and bile can flow through the space between the covering of the stent and the wall of the bile duct [10, 27]. Although regular follow-up CT was not available in our study, the serum bilirubin level decreased significantly 1 week and 1 month after stent placement, regardless of segmental duct blockage. Cholecystitis also did not occur in any patients.
In our study, stent occlusion occurred in nine of 34 patients (26.5 %), mainly due to tumor overgrowth, contrary to others in which sludge formation was the main cause of the stent occlusion [10, 11]. Tumor overgrowth is an uncommon, late complication causing recurrent obstructive symptoms after covered stent placement. It has been reported to occur in patients with longer survival time in the gastroduodenum [28], as was true in our study in which tumor overgrowth occurred late within 64–377 (mean 204) days after stent placement in longer survivals. Recurrent obstructive symptoms cause by stent occlusion can be successfully treated with PTBD or by coaxial placement of a second stent. However, second stent placement can be technically more difficult when bilateral stent in advanced hilar malignancies are placed using the stent-in-stent deployment technique [8], because the wires of the previously inserted stent have a potential risk of hindering the additional stent insertion through the contralateral bile duct [7, 12]. On the contrary, our stent design with side-by-side deployment allows simple placement of an additional stent. Although only two of nine patients with stent occlusion underwent placement of a second stent in our study, once the stent occlusion was negotiated using a hydrophilic guide wire, the procedures were uneventful without difficulty.
Our study is principally limited by a relatively small number of patients and its retrospective design. Further investigation in a large patient group with randomized, prospective design will potentiate strength of this study. Despite the limitation, our results indicate that placement of the Y-shaped branched covered stent seems to be technically feasible and clinically effective for palliative treatment of malignant hilar biliary obstruction. We think that use of this stent may provide effective bilateral internal biliary drainage without interfering the contralateral bile inflow at hilar confluence, long-term stent patency by preventing tumor ingrowth, and a feasible management of stent occlusion. Further investigation with a prospective, randomized trial is needed to validate the advantages of this procedure.
References
Mihalache F, Tantau M, Diaconu B, Acalovshi M. Survival and quality of life of cholangiocarcinoma patients: a prospective study over a 4 year period. J Gastrointestin Liver Dis. 2010;19:285–90.
Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39–47.
LaBerge JM, Doherty M, Gordon RL, Ring EJ. Hilar malignancy: treatment with an expandable metallic transhepatic biliary stent. Radiology. 1990;177:793–7.
Kim CW, Park AW, Won JW, Kim S, Lee JW, Lee SH. T-configured dual stent placement in malignant biliary hilar duct obstructions with a newly designed stent. J Vasc Interv Radiol. 2004;15:713–7.
Ahn SJ, Bae JI, Han TS, et al. Percutaneous biliary drainage using open cell stents for malignant biliary hilar obstruction. Korean J Radiol. 2012;13:795–802.
Gwon DI, Ko GY, Kim JH, et al. Percutaneous bilateral metallic stent placement using a stent-in-stent deployment technique in patients with malignant hilar biliary obstruction. Am J Roentgenol. 2013;200:909–14.
Hwang JC, Kim JH, Lim SG, Kim SS, Yoo BM, Cho SW. Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: prospective long-term study. Scand J Gastroenterol. 2011;46:326–32.
Kim DU, Kang DH, Kim GH, et al. Bilateral biliary drainage for malignant hilar obstruction using the ‘stent-in-stent’ method with a Y-stent: efficacy and complications. Eur J Gastroenterol Hepatol. 2013;25:99–106.
Bae JI, Park AW, Choi SJ, et al. Crisscross-configured dual stent placement for trisectoral drainage in patients with advanced biliary hilar malignancies. J Vasc Interv Radiol. 2008;19:1614–9.
Gwon DI, Ko GI, Yoon HK, et al. Prospective evaluation of a newly designed T-configured stent graft system for palliative treatment of advanced hilar malignant biliary obstructions. J Vasc Interv Radiol. 2010;21:1410–8.
Gwon DI, Ko GI, Yoon HK, et al. Safety and efficacy of percutaneous Y-configured covered stent placement for malignant hilar biliary obstruction: a prospective, pilot study. J Vasc Interv Radiol. 2012;23:528–34.
Naitoh I, Ohara H, Nakazawa T, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24:552–7.
Kang BC, Lee SY, Chung HH. A newly designed Y-shaped covered stent in the palliative treatment of hepatic hilar malignant obstruction: case report. Korean J Radiol. 2013;14:97–101.
Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–202.
Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–34.
Naitoh I, Hayashi K, Nakazawa T, et al. Side-by-side versus stent-in-stent deployment in bilateral endoscopic metal stenting for malignant hilar biliary obstruction. Dig Dis Sci. 2012;57:3279–85.
Gazelle GS, Lee MJ, Mueller PR. Cholangiographic segmental anatomy of the liver. Radiographics. 1994;14:1005–13.
Yasumori K, Mahmoudi N, Wright KC, Wallace S, Gianturco C. Placement of covered self-expanding metallic stens in the common bile duct: a feasibility study. J Vas Interv Radiol. 1993;4:773–8.
Kanasaki S, Furukawa A, Kane T, Murata K. Polyurethane-covered Nitinol Strecker stents as primary palliative treatment of malignant biliary obstruction. Cardiovasc Intervent Radiol. 2000;23:114–20.
Thurnher SA, Lammer J, Thurnher MM, Winkelbauer F, Graf O, Wildling R. Covered self-expanding transhepatic biliary stents: clinical pilot study. Cardiovasc Interv Radiol. 1996;19:10–4.
Han YM, Hwang SB, Lee ST, Lee JM, Chung GH. Polyurethane-covered self-expandable nitinol stent for malignant biliary obstruction: preliminary results. Cardiovasc Interv Radiol. 2002;25:381–7.
Han YM, Kwak HS, Jin GY, Lee SO, Chung GH. Treatment of malignant biliary obstruction with a PTFE-covered self-expandable nitinol stent. Korean J Radiol. 2007;8:410–7.
Tsang TK, Pollack J, Chodash HB. Silicone-covered metal stents an in vitro evaluation for biofilm formation and patency. Dig Dis Sci. 1999;44:1780–5.
Bang BW, Jeong S, Lee DH, Lee JI, Lee SC, Kang SG. The biodurability of covering materials for metallic stents in a bile flow phantom. Dig Dis Sci. 2012;57:1056–63.
Krokidis ME, Hatzidakis AA, Manousaki EG, Gourtsoyiannis NC. Late migration of two covered biliary stents through a spontaneous bilioenteric fistula in a patient with malignant biliary obstruction. Cardiovasc Interv Radiol. 2008;31:222–5.
Kim JW, Gwon DI, Han YM, et al. A prospective, multicenter study of a double stent system for palliative treatment of malignant extrahepatic biliary obstructions. Acta Radiol. 2015;56:1209–15.
Petersen BD, Timmermans HA, Uchida BT, Rabkin JM, Keller FS. Treatment of refractory benign biliary stenoses in liver transplant patients by placement and retrieval of a temporary stent-graft: work in progress. J Vasc Interv Radiol. 2000;11:919–29.
Jang JK, Song HY, Kim JH, Song M, Park JH, Kim EY. Tumor overgrowth after expandable metallic stent placement: experience in 583 patients with malignant gastroduodenal obstruction. Am J Roentgenol. 2011;196:W831–6.
Acknowledgments
We would like to acknowledge So Hee Jung, PhD, for her assistance in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jong Hyouk Yun, Gyoo-Sik Jung, Jung Gu Park, Byung Chul Kang, Dong-Hoon Shin, Byung Chul Yun, and Sang Uk Lee declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yun, J.H., Jung, GS., Park, J.G. et al. Malignant Hilar Biliary Obstruction: Treatment by Means of Placement of a Newly Designed Y-Shaped Branched Covered Stent. Cardiovasc Intervent Radiol 39, 582–590 (2016). https://doi.org/10.1007/s00270-015-1205-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-015-1205-1