Abstract
Purpose
Patients without a competent sphincter of Oddi due to prior surgical or endoscopic therapy are at high risk for liver abscess following chemoembolization despite aggressive antimicrobial prophylaxis. We examined a cohort of such patients undergoing Y-90 resin radioembolization and compared them to a cohort of chemoembolized patients.
Methods
Review of our quality-assurance database identified 24 radioembolizations performed in 16 patients with prior biliary intervention. An aggressive prophylactic regimen of oral levofloxacin and metronidazole 2 days pre-procedure continuing for 14 days after, oral neomycin/erythromycin bowel prep the day before, and IV levofloxacin/metronidazole the day of treatment was prescribed. Patients underwent resin microsphere radioembolization dosed according to the BSA method. Patients had clinical, imaging, and laboratory assessment 1 month after each treatment, and then every 3 months. The chemoembolization cohort consisted of 13 patients with prior biliary intervention who had undergone 24 chemoembolization procedures.
Results
No radioembolization patient developed an abscess. In the cohort of chemoembolized patients who received the same prophylaxis, liver abscess occurred following 3 of 24 (12.5 %) procedures in 3 of 13 (23 %) patients, one fatal.
Conclusions
This preliminary experience suggests that the risk of liver abscess among patients with prior biliary intervention may be lower following radioembolization than chemoembolization, which could potentially expand treatment options in this high-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radioembolization and chemoembolization are common palliative treatments for unresectable hepatic malignancies. Chemoembolization combines ischemia from embolization with local delivery of chemotherapeutic drugs, whereas yttrium-90 (Y-90) radioembolization relies primarily on beta-isotope emission and selective internal radiation of the tumor.
Abscess formation is a rare complication following chemoembolization, but risk of liver abscess has been reported to increase 800-fold in patients with a biloenteric anastomosis or stent [1, 2]. This risk was shown to decrease somewhat but remain significantly elevated despite aggressive antibiotic prophylaxis [3]; hence, prior biliary intervention remains a relative contraindication to chemoembolization.
The purported mechanism for abscess formation is microscopic ischemic injury of the colonized bile ducts. The endpoint of radioembolization is delivery of a prescribed dose of Y-90, without vascular stasis. We hypothesized that there may be less risk of bile duct injury and consequent abscess formation with radioembolization than has been observed with chemoembolization. This study compares the rate of abscess formation in patients with prior biliary anastomosis or stent treated with Y-90 radioembolization versus a reference cohort treated with chemoembolization.
Materials and Methods
Sample Size for Hypothesis Testing
In our previously reported series, abscesses occurred in approximately 30 % of patients following chemoembolization despite aggressive antibiotic prophylaxis [3]. To calculate a sample size, radioembolization with aggressive antibiotic prophylaxis was posited to have a liver abscess rate of 5 % or less. To detect this difference relative to chemoembolization with 80 % power would require a sample size of 28 patients, which is larger than all prior publications on this topic and was recognized not to be a realistic goal at a single center. Colleagues at a number of other major radioembolization centers were polled to see if there was sufficient experience for a pooled multicenter report, but none had substantial experience with this patient population. Recognizing these limitations, a retrospective cohort comparison was performed.
Radioembolization Cohort
Under institutional review board approval, we reviewed our Quality Assurance Database (HI-IQ, ConexSys, Lincoln, RI) and identified 24 radioembolization procedures in 16 patients with prior biliary intervention (range, 1–4 procedures per patient). Nine patients had metastasis from pancreatic neuroendocrine tumors, four patients had metastatic cholangiocarcinoma, two patients had metastatic pancreatic or ampullary adenocarcinomas, and one metastatic rectal carcinoma. Nine had a prior Whipple resection, five patients had prior ERCP with biliary stent placement, and two patients had received bile duct reconstruction following Roux-en-Y hepatojejunostomy. No patients had a history of liver transplantation, neutropenia, or were otherwise immunocompromised. Baseline clinical and laboratory data are summarized in Table 1.
Pretreatment mesenteric angiography was performed on each patient, including Tc99-MAA injection to identify unwanted shunting to the lungs or gastrointestinal tract. Comprehensive metabolic panel, complete blood count, coagulation profiles, and serum tumor markers were obtained within 14 days of the procedure.
Based on previous studies showing high risk of abscess formation following chemoembolization in patients with prior biliary intervention, an aggressive prophylactic regimen was used. The regimen began with oral levofloxacin 500 mg daily and metronidazole 500 mg twice daily starting 48 h before the procedure. One gram of neomycin and 1 g of erythromycin base were administered at 1, 2 and 11 p.m. on the day before the procedure. IV levofloxacin and metronidazole were administered on the day of radioembolization and were continued orally for 2 weeks after discharge.
Radioembolization was performed using Y-90 resin microspheres at a lobar dose calculated with the BSA method, adjusted for tumor burden, according to the manufacturer’s instructions for use. Patients with bilobar disease were treated in a sequential lobar fashion with 30–60 days between treatments. All patients were evaluated 1 month, 3 months, and then every 3 months following treatment. Follow-up visits consisted of CT or MRI studies and clinical and laboratory toxicity assessment, using the same serum tests obtained at baseline. Patients with evidence of incomplete tumor targeting or subsequent intrahepatic disease progression were treated again. Liver abscess formation was diagnosed based on a combination of clinical presentation of fever, pain, leukocytosis occurring more than 1 week after radioembolization, evidence of abscess formation on imaging, and aspiration of pus with positive cultures.
Chemoembolization Cohort
The reference cohort consisted of 13 patients who underwent 24 chemoembolization procedures (range 1–4), including seven patients from our previously reported series [3]. Among these were three with hepatocellular carcinoma, three with cholangiocarcinoma, two each with metastatic rectal cancer or pancreatic cancer, and one each with metastatic neuroendocrine tumor, renal cell carcinoma, or ovarian cancer. Five had Whipple resections; the others had biliary stents. One of the HCC patients had undergone liver transplantation; the remainder was not immunocompromised. The reference group received the same aggressive prophylactic therapy followed by chemoembolization with 100 mg of cisplatin, 50 mg of doxorubicin, and 10 mg of mitomycin-C dissolved in 10 mL of iodinated contrast medium and emulsified with 10 mL of iodized oil and 150–250 µm of polyvinyl alcohol particles or 100–300 micron trisacryl microspheres (Embospheres, Biosphere Medical) in a lobar distribution until near stasis (“tree-in-winter”) was achieved. Chemoembolization was repeated monthly as needed until the entire tumor burden was treated. Patients underwent clinical, laboratory, and imaging follow-up as described for the radioembolization cohort.
An odds ratio was calculated and compared with the odds ratio of liver abscess formation in patients treated with chemoembolization with use of the Fisher exact test. P value of 0.05 was considered the threshold for statistical significance.
Results
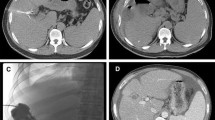
Among the 16 radioembolization patients, no abscesses occurred following 24 procedures (0 %). In the reference cohort, liver abscesses occurred following chemoembolization in 3 of 13 patients (23 %) among a total of 24 procedures (12.5 %) despite aggressive prophylactic therapy. One abscess occurred after the patient’s fourth chemoembolization. Liquefaction with gas was first detected on CT examination 1 month after this procedure but was initially felt to be tumor necrosis. Three months after the chemoembolization procedure, this area was diagnosed as an abscess and drained, despite which the patient succumbed. The second abscess occurred after the second chemoembolization in another patient who had not taken the pre-procedure oral antibiotics for that specific procedure but did receive the intravenous and post-procedure antibiotics per protocol. He developed a liver abscess 3 months after chemoembolization, which was drained. The third patient developed an abscess 2 months after his first chemoembolization, which was drained.
The Fisher exact test was performed to compare the occurrence of liver abscesses in the group treated with aggressive prophylactic therapy and chemoembolization versus the group treated with aggressive prophylactic therapy and radioembolization. Radioembolization showed a statistical trend toward a decrease in liver abscess formation on a per-patient basis (P = 0.078), less so on a per-procedure basis (P = 0.23).
Discussion
Liver abscess formation is a rare but significant complication of chemoembolization, a risk that is higher in patients with prior biliary interventions [4–7]. Mehzir reported 14 abscesses among 971 patients undergoing 2,045 liver embolizations; 13 of 14 had a compromised sphincter of Oddi [4]. Antibiotic prophylaxis in this series consisted of three doses of a first-generation intravenous cephalosporin in patients with a competent sphincter of Oddi, and 5 days of intravenous piperacillin/tazobactam or cefotetan in patients with a bilioenteric anastomosis or stent. Woo [7] reported 25 patients with a biloenteric anastomosis who received a total of 65 chemoembolizations. Twelve patients (48 %) developed a liver abscess; two of which were fatal. Antibiotic prophylaxis was not used in most of these patients. Kim et al. reported abscess formation in only one of 150 (0.7 %) patients without bilioenteric anastomosis but in six of seven patients (86 %) with history of a prior Whipple procedure [2].
Aggressive prophylactic antimicrobial therapy has been used to mitigate the risk of developing liver abscesses following chemoembolization among patients with prior biliary intervention. Patel et al. [3] reported two liver abscesses among seven patients (28.6 %; 16 total procedures, 12.5 %) following 2 weeks of antibiotic prophylaxis with levofloxacin and metronidazole beginning 2 days before chemoembolization. Khan [8] reported no abscesses among ten patients who received 25 chemoembolizations treated with moxifloxacin 400 mg orally starting 3 days before the procedure and continued for 17 days after. The Society of Interventional Radiology Quality Improvement Guidelines for Chemoembolization and Practice Guidelines for Adult Antibiotic Prophylaxis both recommend more potent and protracted antibiotic therapy in this patient population while acknowledging the relatively weak level of evidence supporting the practice [9, 10].
The pathophysiology behind abscess formation following chemoembolization is still unclear. It is believed that patients with prior biliary anastomoses have retrograde intestinal bacterial colonization of the biliary tree. Chemoembolization results in embolization of the peribiliary capillary plexus, which arises from the hepatic artery and provide the primary vascular supply to the biliary ducts. Histologic examination of the liver in patients with hepatocellular carcinoma treated with transarterial therapy show moderate to marked obliteration of the peribiliary capillary plexus in 54–78 % of cases with gross biliary necrosis or biloma formation in 12.5 % of livers [11]. A combination of both increased bacterial colonization as well as bile duct ischemia following chemoembolization offers mechanism for the increased risk of abscess in this patient population.
In contrast, preclinical studies of embolization with resin microspheres in a swine model showed no bile duct injury on histologic examination [12]. An angiographic study of patients undergoing radioembolization with glass microspheres found no macroscopic evidence of embolic effect, such as pruning, stasis, or vessel cutoff; in fact, readers were unable to distinguish preembolization and postembolization angiograms in the majority of patients [13]. Radioembolization with glass microspheres has been shown to be safe in the setting of biliary obstruction among patients without prior biliary intervention [14]. To our knowledge, only two cases of liver abscess following radioembolization have been reported: one in a patient with a prior Whipple resection, and one without prior biliary intervention or obstruction [15].
The results of this study are important, because they suggest that with aggressive antibiotic prophylaxis, radioembolization may have a lower risk of abscess in patients with prior biliary intervention. No patients in our cohort developed an abscess. However, the sample size in the trial remains a major limitation. While large relative to all previous reports of patients with prior biliary intervention undergoing hepatic embolization, it is still relatively small relative to the sample size required for adequate statistical power to draw firmer conclusions. This limits the strength of our analysis, since it offers only slightly better than 50 % power to detect the posited reduction in abscess rate at α = 0.05.
Our data also suggest that radioembolization is no worse than chemoembolization in terms of incidence of abscess formation. This needs to be confirmed by larger experience among patients with prior biliary intervention. Furthermore, the necessity for aggressive antibiotic prophylaxis can now be reconsidered. Because we saw no abscesses with aggressive prophylaxis, it would be reasonable to investigate radioembolization with standard (or no) antibiotic prophylaxis.
References
de Baere T, Roche A, Amenabar JM et al (1996) Liver abscess formation after local treatment of liver tumors. Hepatology 23:1436–1440
Kim W, Clark TW, Baum RA et al (2001) Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol 12:965–968
Patel S, Tuite CM, Mondeschein JI, Soulen MC (2006) Effectiveness of an aggressive antibiotic regimen for chemoembolization in patients with previous biliary intervention. J Vasc Interv Radiol 17:1931–1934
Mehzir JJ, Fong Y, Fleischer D et al (2011) Pyogenic abscess after hepatic artery embolization: a rare but potentially lethal complication. J Vasc Interv Radiol 22:177–182
Song SY, Chung JW, Han JK et al (2001) Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical response. J Vasc Interv Radiol 12:313–320
Ong GY, Changchien CS, Lee CM et al (2004) Liver abscess complicating transcatheter arterial embolization: a rare but serious complication. A retrospective study after 3878 procedures. Eur J Gastroenterol Hepatol 16:737–742
Woo S, Chung JW, Hur S et al (2013) Liver abscess after transarterial chemoembolizations in patients with biloenteric anastomosis: frequency and risk factors. Am J Roentgenol 200:1370–1377
Khan W, Sullivan KL, McCann JW et al (2011) Moxifloxacin prophylaxis for chemoembolizations or embolizations in patients with previous biliary interventions: a pilot study. AJR 197:W343–W345
Brown DB, Nikolic B, Covey AM et al (2012) Quality improvement guidelines for transhepatic arterial chemoembolization, embolizations, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 23:287–294
Venkatesan AM, Kundu S, Sacks D et al (2010) Practice guidelines for adult antibiotic prophylaxis during vascular and interventional procedures. J Vasc Interv Radiol 21:1611–1630
Kobayashi S, Nakanuma Y, Terada T, Matsui O (1993) Postmortem survey of bile duct necrosis and biloma in hepatocellular carcinoma after transcatheter arterial chemoembolization therapy: relevance to microvascular damages of peribiliary capillary plexus. Am J Gastrol 88:1410–1415
Bilbao JI, de Martino A, de Luis E et al (2009) Biocompatibility, inflammatory response, and recanalization characteristics of nonradioactive resin microspheres: histologic findings. Cardiovasc Interv Radiol 32:727–736
Sato K, Lewandowski RJ, Bui JT et al (2006) Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (Theraspheres®): assessment of hepatic arterial embolization. Cardiovasv Interv Radiol 29:522–529
Gaba RC, Riaz A, Lewandowski RJ et al (2010) Safety of yttrium-90 microsphere radioembolization in patients with biliary obstruction. J Vasc Interv Radiol 21:1213–1218
Mascarenhas NB, Mulcahy MF, Lewandowski RJ, Salem R, Ryu RK (2010) Hepatic abscess after yttrium-90 radioembolization for islet-cell tumor hepatic metastasis. Cardiovasc Interv Radiol 33:650–653
Conflict of interest
Michael Soulen is a paid speaker and proctor for Sirtex Medical; Aurada Cholapranee, Diana van Houten, Ginna Deitrick, Mandeep Dagli, Deepak Sudheendra, Jeffrey I. Mondschein: no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cholapranee, A., van Houten, D., Deitrick, G. et al. Risk of Liver Abscess Formation in Patients with Prior Biliary Intervention Following Yttrium-90 Radioembolization. Cardiovasc Intervent Radiol 38, 397–400 (2015). https://doi.org/10.1007/s00270-014-0947-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0947-5