Abstract
Purpose
To evaluate the short-term safety and efficacy of the new generation of 70–150 µm drug-eluting beads (M1 DEB) in patients with hepatocellular carcinoma undergoing transarterial chemoembolization (TACE) as a primary therapy or as a bridge to liver transplantation (LT).
Methods
Forty-five consecutive patients underwent TACE with M1 DEB loaded with doxorubicin (DEBDOX/M1). Clinical data were recorded at 12, 24, and 48 h, 7 and 30 days after treatment. Response was assessed by computed tomographic scan according to the modified response evaluation criteria in solid tumors criteria, and a second DEBDOX/M1 TACE was scheduled within 6 weeks in case of a noncomplete response.
Results
All patients had well-compensated cirrhosis (97.7 % Child A, 44.4 % hepatitis C virus, median age 61 years). Twenty patients (44.4 %) had Barcelona Clinic for Liver Cancer class B disease; the median number of nodules and their sum of diameters were 2 (range 1–6) and 43 mm (range 10–190), respectively. The mean number of TACE procedures per patient was 1.4. Objective response rate (complete + partial response) was 77.7 % with a median time to best response of 3 months (95 % confidence interval 2–4). In 13 patients, DEBDOX/M1 TACE served as a bridge/downstaging to LT/surgery. Pathology showed that more than 90 % necrosis was achieved in 10 of 28 nodules. DEBDOX/M1 TACE was well tolerated, and the grade 3/4 adverse event rate was low (1 of 65 procedures).
Conclusion
DEBDOX/M1 TACE is an effective procedure with a favorable safety profile and promising results in terms of objective response rate, tumor downstaging, and necrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transarterial chemoembolization (TACE) is the standard of care for early–intermediate hepatocellular carcinoma (HCC) not amenable to curative treatments and also as a bridge to liver transplantation (LT). TACE achieves its effect by inducing tumor ischemia and enhancing the cytotoxic action of chemotherapeutic agents, allowing a prompt and long-lasting intratumoral retention.
Randomized studies have demonstrated prolonged survival in properly selected patients treated with TACE using Lipiodol (conventional TACE) compared to best supportive care [1]. In the last few years, drug-eluting beads (DEB) for the transarterial treatment of HCC have been developed to actively load doxorubicin hydrochloride (DEBDOX) from its solution and release it in a controlled and prolonged fashion in the tumor’s arterial network [2, 3]. However, technical aspects of the procedure, such as drugs to be loaded, dimensions of the DEB, and different technical approaches, are still under debate [4, 5].
Although the first published series showed interesting results in terms of radiologic response and safety profile [6], a recent multicenter randomized controlled trial comparing DEB–TACE (using 300–500 μm particles) versus conventional TACE found no significant difference in the objective response rate between the two approaches [7]. However, additional studies proved that smaller-diameter DEB may cause a significant and extensive tumor necrosis [8, 9], and their effect on response and survival rates is currently being investigated in clinical trials. Recently, a newer generation of DEBDOX particles called M1 (DC bead, Biocompatibles, Surrey, UK) with a diameter between 70 and 150 µm has been developed. Preliminary studies in animal models showed that this new generation of particles may allow a more concentrated drug delivery as well as greater tumor penetration and devascularization while uploading the same amount of doxorubicin as do larger particles [10].
The aim of this prospective pilot study was to evaluate the efficacy and safety of this new generation of DEB in a consecutive series of patients with early/intermediate unresectable HCC.
Materials and Methods
The study included 45 consecutive HCC patients recruited from November 2012 to September 2013 at our institution. Approval of the local ethical committee was obtained, and all patients provided informed consent before being enrolled onto the study.
Patients with a diagnosis of HCC were discussed at the institution’s multidisciplinary liver tumor board, whose members include several professionals with a long and dedicated experience in the field, such as radiologists, hepatologists, pathologists, surgeons, and medical oncologists.
Inclusion criteria were as follows: age > 18 years; diagnosis of HCC by means of noninvasive criteria according to European Association for the Study of the Liver guidelines [11] or histology; early/intermediate stage HCC according to the Barcelona Clinic for Liver Cancer (BCLC) classification [12] not amenable to resection/ablation; well-compensated cirrhosis with a Child-Pugh score up to B7; and Eastern Cooperative Oncology Group [13] performance status of 0. Exclusion criteria were as follows: bilirubin levels greater than 3 mg/dL; main or bland portal vein thrombosis; previous palliative treatments for HCC (such as TACE, radiotherapy, or sorafenib); contraindications for embolization (e.g., arteriovenous shunting considered clinically significant and not amenable to correction by embolization); and intolerance to doxorubicin (leucocyte count of <3,000/mm3 or cardiac ejection fraction of <50 %).
All patients underwent computed tomographic (CT) scan of thorax and abdomen to assess tumor burden and hepatic extension, to better define the vascularization network of the tumoral area, and to exclude extrahepatic spread and vascular invasion. Patients were imaged with a dual-source, dual-energy CT scanner (Somatom Definition Flash, Siemens Medical Solutions, Germany). All CT scans, before and after treatment, were performed with the same protocol with and without 120–140 mL of intravenous contrast medium (Iopamiro 370, Bracco, Milan, Italy) injected at a rate of 4 mL/s using the bolus tracking technique with image acquisitions in the arterial, portal, and late phases.
Diagnostic visceral angiography (Siemens Axiom Angiographic Unit, Erlangen, Germany) of both the celiac trunk and the superior mesenteric artery was first performed with a 5F catheter (Cobra or Simmons 1 shaped, Imager II, Boston Scientific MA, USA) to investigate the arterial supply of the liver. Chemoembolization was performed as selectively as possible with a coaxial 2.7F microcatheter (Progreat, Terumo, Japan). When a superselective approach to the target nodules was not feasible, TACE was performed by including a maximum of three segments per session. TACE was performed with 2 mL of M1 DEB (70–150 µm particles) loaded with 75 mg of doxorubicin (Adriblastina, Pfizer, New York, NY, USA) (DEBDOX/M1) and with no adjustments for body surface. DEB were diluted in 20–30 mL of contrast medium (Iopamiro-370, Bracco, Milan, Italy) and injected until near stasis was observed in the artery directly feeding the tumor (i.e., the contrast column should clear within 2–5 heartbeats) [4]. Hemostasis was done with an Exoseal (Cordis, Miami Lakes, FL, USA) device. Premedication consisted of 30 mg of ketorolac (Toradol, Recordati S.p.A., Milan, Italy), 4 mg of ondansetron (Ondansetron, Fresenius Kabi, Verona, Italy), and 1 g of paracetamol (Paracetamolo, S.A.L.F. S.p.A., Bergamo, Italy) provided at the beginning of the procedure. No antibiotic prophylaxis was provided. Patients were hospitalized and followed until discharge. Toxicity data were recorded at 12, 24, and 48 h, 7 and 30 days after treatment and included patient’s clinical examinations, laboratory data, and registration of adverse events according to the Common Terminology Criteria for Adverse Events, version 4.0 [14].
Tumor response was assessed by means of the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria 4 weeks after the procedure, then every 3 months thereafter [15]. A further procedure was scheduled within 2 weeks from the radiological evaluation in case of noncomplete response or disease progression as long as the patient still fit the inclusion criteria.
All evaluations were done on the basis of CT scan imaging. All procedures and imaging evaluations were performed by two expert radiologists. In case of disagreement, a third radiologist was asked to review the CT scans until consensus was obtained.
To better evaluate the efficacy of DEBDOX/M1 TACE, treated lesions were examined for necrosis at pathology (gold standard) in patients who underwent transplantation or resection after TACE.
Descriptive statistical analysis was carried out as median and range for continuous variables and percentage for categorical data. Time to best response was computed from the time of the first DEBDOX/M1 TACE treatment by Kaplan–Meier methodology. Calculations were performed by SPSS 19 software (IBM, Armonk, NY, USA).
Results
Table 1 summarizes the patients’ characteristics. Patients were primarily men (77.8 %) aged 18–95 years (median 61 years). All patients were asymptomatic (performance status 0), and cirrhosis was primarily related to hepatitis C virus (44.4 %). The proportion of patients in the BCLC A and B stages was identical (both 44.4 %). Almost all patients had Child-Pugh class A disease (97.7 %). Less than half of the patients were affected by other collateral diseases (mainly diabetes and arterial hypertension). Most patients (66.6 %) had undergone previous curative treatments for HCC (i.e., resection or ablation) and were offered DEBDOX/M1 TACE for recurrent HCC. The median number of nodules was 2 (range 1–6) with a median maximum diameter of 27 mm (range 9–143 mm). Median sum of diameters was 43 mm (range 10–190 mm).
All chemoembolization procedures were successful (i.e., chemoembolization to the target tumor was always performed), and no technical complications were observed. The overall total number of the performed DEBDOX/M1 TACE procedures was 65 (22 segmental, 34 bisegmental, 9 trisegmental); 29 patients (64.4 %) underwent only one procedure, 12 (26.6 %) received two, and 4 (9 %) three. The mean number of TACE procedures per patient was 1.4.
The best response achieved per patient, evaluated according to mRECIST criteria, is shown in Fig. 1. Fifteen patients experienced a complete response (33.3 %) and 20 (44.4 %) a partial response, accounting for a 77.7 % objective response rate (complete response + partial response). The analysis per nodule demonstrated an objective response (complete response + partial response) in 78 % of the 103 nodules (complete response in 42 %) (Table 2). Median time to best response was 3 months (95 % confidence interval 2–4).
Thirteen patients underwent surgery after 20 sessions of DEBDOX/M1 TACE: 12 patients underwent LT after a cumulative number of 19 procedures (Fig. 2), and 1 patient underwent a major hepatectomy. Median time between DEBDOX/M1 TACE and LT was 3.5 months. The histological analysis of 28 nodules in the 13 available specimens showed an increase of 90 % necrosis in 10 cases (100 % in 7 cases) (Table 3).
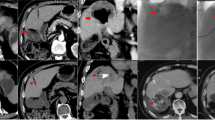
Radiological and histological response before and after DEBDOX/M1 TACE in a transplanted patient. A Pretreatment arterial phase CT scan; the arrow shows a nodule of HCC in segment VI. B Follow up CT scan obtained 35 days after DEBDOX/ M1 TACE; the arrow indicates a complete radiological response of the nodule. C Histological specimen that shows complete necrosis and deep penetration of the beads in the neoplastic vessels
Toxicity data are reported in Table 4. Among mild (grade 1/2) adverse events, the most common were postembolic syndrome and abdominal pain (4.4 % each). The only serious adverse event (grade 3) reported was a case of bleeding from esophageal varices, caused by the worsening of the portal hypertension; the patient had a short hospitalization and fully recovered within 1 month. A grade 1/2 increase in bilirubin levels was the most common (6.6 %) altered laboratory value reported after DEBDOX/M1 TACE.
Discussion
TACE is considered the treatment of choice in patients with a well-preserved liver function and early/intermediate stage HCC not amenable to curative treatments [11, 16]. To improve the impact of TACE on survival, new approaches should be developed in order to increase the antitumoral effect, prolong the duration of response, and maintain the lowest adverse event rate. Chemoembolization with DEB and doxorubicin exhibited good tolerability with minimal postembolization symptoms and systemic toxicity because it combines local cytotoxicity (with low systemic levels of doxorubicin) and ischemia [2, 3].
Because the diameter of the beads seems to be correlated with the beads’ therapeutic action [4, 22], pharmaceutical research has been directed toward the production of smaller particles aiming at a deeper penetration within the arterioles of the tumor network. Recently a new generation of beads (M1) has been introduced into the locoregional treatment of HCC, but data about their efficacy and safety profile are lacking. To our knowledge, this is the first pilot study evaluating the efficacy and safety of M1 DEB for the treatment of HCC. The results of this prospective study demonstrate a promising radiological response rate, with an objective response rate of 77.7 %. This result is slightly higher than that reported in the PRECISION V trial [7] and is in line with other series using particles of greater size [17–19].
In 13 of 45 patients, TACE with M1 allowed a bridging/downstaging to radical surgery (LT in 12 patients and a major resection in 1 patient). The good results in terms of radiological response were also confirmed by the explant pathological data (necrosis greater than 90 % in 10 of 28 nodules and an histologically proven complete necrosis in 25 % of the nodules examined). These results are in line with those reported in other series [20].
The best radiological response was obtained in 29 patients after the first cycle of TACE, in 12 patients after the second, and in 4 patients after the third. This seems to support the concept of repeating TACE on demand whenever the result after the first cycle is suboptimal, with the aim of obtaining complete necrosis after two or three procedures and modulating the number of cycles on the basis of the obtained radiological response.
Recorded toxicity was limited. The low incidence of an increase in the levels of transaminases probably reflects the high selectivity of the procedure and of the particles’ deposition. Overall, the procedures were well tolerated, and toxicities were lower than those reported for larger particles in recent publications [21] and in line with recent results reported with particles 30–60 µm in size [22]. No relevant systemic adverse effects related to the release of doxorubicin into the bloodstream were observed, which is in line with the results published elsewhere with larger particles [7, 19].
The excellent tolerability and the low incidence of adverse effects in our series also confirm the importance of performing chemoembolization procedures in a selective manner with the use of a microcatheter to avoid imbalancing liver function and to optimize the treatment of the tumor nodules. No cases of liver abscesses were reported—a finding that is relatively frequent in other series; nor were cases of cholecystitis or alterations of vessels walls and structures apparent. These data are important if TACE is put into the perspective of being a bridging/downstaging treatment option to curative treatments such as resection and LT.
To our knowledge, this is the first study regarding the use of M1 particles in HCC. The study had a prospective design in order to collect data accurately and objectively. The results are complete for all 45 patients enrolled, and for 13 of them, a histologically proven response to TACE on surgical specimen is available. This study has some drawbacks that may represent limitations. These include the small number of patients and the short follow-up time, which did not allow us to assess the impact of TACE with M1 particles on patient survival or obtain data on time to disease progression.
In conclusion, the results of this pilot study indicate that DEBDOX TACE with M1 DEB is a safe and effective procedure in unresectable HCC as a primary therapy or as a bridge to LT. Further larger and randomized studies comparing M1 DEB with larger particles are necessary to confirm our results.
References
Llovet JM, Bruix J (2003) Systemic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37:429–442
Poon RT, Tso WK, Pang RW et al (2007) A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 5:1100–1108
Varela M, Real MI, Burrel M et al (2007) Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 46:474–481
Lencioni R, de Baere T, Burrel M et al (2012) Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC beads (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol 35:980–985
Lencioni R, Crocetti L (2010) Loco-regional treatment of hepatocellular carcinoma. Radiology 262:43–58
Malagari K, Alexopoulou E, Chatzimichail K et al (2008) Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC beads. Abdom Imaging 33:512–519
Lammer J, Malagari K, Vogl T et al (2010) Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 33:41–52
Nicolini A, Martinetti L, Crespi S et al (2010) Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol 21:327–332
Bonomo G, Pedicini V, Monfardini L et al (2010) Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Intervent Radiol 33:552–559
Geschwind J (2012) Doxorubicin eluting bead sizes 100–300 μm and 70–150 μm in the VX2 model. Paper presented at: ECIO 2012 (Terumo Symposium)
EASL-EORTC (2012) Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
National Cancer Institute (2009) Common terminology criteria for adverse events v4.0. NCI, NIH, DHHS. May 29, 2009. NIH publication 09-7473
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Bruix J, Sherman M (2011) Practice guidelines committee, American Association for the Study of Liver Disease. Management of hepatocellular carcinoma. An update. Hepatology 53:1020–1022
Burrel M, Reig M, Forner A et al (2012) Survival of patients with hepatocellular carcinoma treated by transarterial embolization (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 56:1330–1335
Malagari K, Pomoni M, Moschouris H et al (2012) Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol 35:1119–1128
Jun Song M, Chun HL, Song DS et al (2012) Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 57:1244–1250
Nicolini D, Svegliati-Baroni G, Candelari R et al (2013) Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol 19:5622–5632
Sacco R, Bargellini I, Bertini M et al (2011) Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 22:1545–1552
Malagari K, Pomoni M, Moschouris H et al (2014) Chemoembolization of hepatocellular carcinoma with Hepasphere 30–60 µm. Safety and efficacy study. Cardiovasc Intervent Radiol 37:165–175
Conflict of interest
Carlo Spreafico, Tommaso Cascella, Antonio Facciorusso, Carlo Sposito, Rodolfo Lanocita, Carlo Morosi, Enrico Maria Civelli, Marta Vaiani, Sherrie Bhoori, Alessandro Pellegrinelli, Alfonso Marchianò, and Vincenzo Mazzaferro declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spreafico, C., Cascella, T., Facciorusso, A. et al. Transarterial Chemoembolization for Hepatocellular Carcinoma with a New Generation of Beads: Clinical–Radiological Outcomes and Safety Profile. Cardiovasc Intervent Radiol 38, 129–134 (2015). https://doi.org/10.1007/s00270-014-0907-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-014-0907-0