Abstract
Purpose
To evaluate the long-term effects of radiofrequency ablation (RFA) of renal masses (RM) and compare them with surgery.
Methods
A total of 203 RM (193 malignant; mean size 30 mm) in 137 patients (95 male subjects; average age 64 years) underwent RFA. Complications and technique effectiveness were evaluated. Overall survival, cancer-specific survival, and disease-free survival were calculated (mean follow-up time 39 months). Predictors for complications, technique effectiveness, and survival were investigated.
Results
Seventeen (8.4 %) adverse events were recorded (2 % major complications). Exophytic development and smaller size were protective against adverse events. Complete ablation was obtained in 87 % RM (93 % ≤3 cm, 89 % ≤4 cm). T1a threshold was a positive predictor for complete ablation and central location a negative one. Three- and 5-year overall survival were 84 and 75 %; cancer-specific survival 96 and 91 %; and disease-free survival 80 and 75 %. Considering only the 79 patients with newly diagnosed renal cell carcinoma, T1a disease stage resulted a positive predictor for both overall survival (87 and 83 % at 3 and 5 years) and cancer-specific survival (100 % at 5 years).
Conclusion
RFA of noncentral small RM is safe and effective, and it provides favorable long-term oncological outcomes. Selection criteria for RFA can also include T1a renal cell carcinoma in patients without surgical contraindications, even though randomized controlled trials are needed to establish the best treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the first report of a radiofrequency ablation (RFA) of a renal cell carcinoma (RCC) in 1999 [1], the use of ablative therapies of renal masses (RM) is hugely increased. During the last decade, safety has been established, and larger series have provided data on oncological outcomes.
Having contributed to the development of renal RFA since the early 2000s, we can now evaluate our long-term results and thus add our series to the literature. It could be useful to update the indications of RFA in the management of RM as we await randomized controlled trials that could definitely compare RFA to the standard therapies. Thus, our aim was to analyze the variables that already allow a proper patient selection and to eventually identify the parameters that would permit randomized controlled trials comparing it with surgery.
Materials and Methods
Data were retrieved from a consecutive database of 1,670 RFA patients since December 2000 (renal patients 162 of 1,670, 9.7 %; 234 RM overall). A retrospective analysis was conducted on the 137 patients (203 RM) treated up to December 2011 (mean follow-up time 39 months).
The study was carried out in accordance with the guidelines established by the institutional review board of our institution and with the Helsinki criteria.
Patients and Tumors
Each patient came to treatment on the basis of a clinical indication from a multidisciplinary board assessment (urologists, interventional radiologists, oncologists, and anesthesiologists). The RFA option was preferred according to standard indications (patients with contraindications to surgery, solitary kidney, hereditary tumors, or bilateral tumors) or other criteria (e.g., rejection of surgery, recurrence after resection, slow-growing RM in elderly patients, patients with transplanted kidney) [2, 3].
Ninety-five patients were men (69 %) and 42 were women, with an average age of 64 years (range 20–88 years). Nine patients (6.6 %) had benign tumors (8 oncocytomas and 1 angiomyolipoma), 125 (91.2 %) RCC, and 3 (2.2 %) metastases (2 from lung cancer, 1 from salivary gland cancer). Thirteen patients (9.5 %) had hereditary RCC: 11 von Hippel-Lindau and 2 hereditary papillary RCC (HPRCC). Thirty-one patients (22.6 %) had solitary kidney: 2 (1.5 %) transplant recipients, 4 (2.9 %) for reasons other than neoplasms, 25 (18.2 %) for previous RCC. Patients with previous RCC (including the 25 with previous radical nephrectomy) numbered 46; a total of 39 had had only renal tumors (in the contralateral and/or in the same kidney), with 7 also having positive lymph nodes or metastases. Of the 79 patients with newly diagnosed RCC (excluding the 9 with benign RM and the 3 with metastases, but including the 46 with other RCC before RFA), 67 patients had stage T1a tumors and 11 had stage T1b tumors; 1 had a T2 RCC.
The 203 RM included the following: 193 malignancies (126 clear cell, 24 von Hippel-Lindau, 20 papillary, 14 HPRCC, 3 chromophobe, 6 metastases) and 10 benign (9 oncocytomas, 1 angiomyolipoma). The average diameter was 3 cm (12–75 mm). Seventy (35 %) were >3 cm, 133 (65 %) ≤3 cm, 180 (88 %) ≤4 cm, and 23 (12 %) >4 cm. RM were unique in 118 cases (58 %) and multiple in 85 (42 %). A total of 123 RM were exophytic, 12 central, 67 parenchymal, and 1 mixed (according to the classification of Gervais et al. [4]). Thirty-nine RFA in 15 patients were performed in a kidney that was already subject to partial nephrectomy (PN) (Table 1).
Preprocedural Evaluation
Before ablation, contrast computed tomography (CT) or magnetic resonance imaging (MRI) were always performed, as was a pathologic diagnosis. In particular, a core biopsy was performed in all patients with other known extrarenal neoplasms or with an indeterminate mass present at imaging [5–7].
Coagulation Tests and Renal Function were Assessed
Indications as well as potential benefits and risks of the procedure were discussed with the patients, all of whom signed an informed consent form.
Procedures
All RFA were performed percutaneously in an analgosedation regimen, mostly with ultrasound (US) targeting and monitoring (only 7 under CT guidance, 3.5 %). Transperitoneal approaches were also considered (19, including 10 transhepatic approaches to RM on the anteromedial side of the right kidney). To better visualize poorly vascularized parenchymal RM (typically papillary RCC), contrast-enhanced US (SonoVue, Bracco, Milan, Italy) was utilized during the insertion of the electrodes. Multi-tined needles were used (Rita Medical Systems, USA, 95 cases; Boston Scientific, USA, 69; Meditalia Biomedica, Italy, 39), following the protocols recommended by the manufacturers.
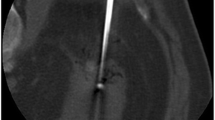
In case of relatively higher risk of injuries to contiguous structures (e.g., colon, upper urinary tract), adjunctive techniques were adopted (electrode torquing, whenever needed; 10 hydrodissection; 3 pyeloperfusion during ureteroscopy; Fig. 1).
RFA of a centrally located RM. A Percutaneous US-guided insertion of a multi-tined electrode-needle, B fluoroscopy during ureteroscopy, and C pyeloperfusion showing a hooked electrode (arrow) protruding through the pyelic wall (then retracted). D Follow-up CT urography demonstrating the CA of the RCC without injuries to the collecting system
The patients remained under observation overnight and were discharged the next day.
Adverse Events
All adverse events (AE) were recorded. Major complications (MC) were identified on the basis of the Society of Interventional Radiology criteria [2].
The search for possible predictors for AE and MC considered the following: solitary kidney, size, side, location and development of the RM (exophytic, central, parenchymal, or mixed), guidance (US vs. CT), transperitoneal approach, previous PN, and treatment of multiple RM in the same session.
Follow-Up
The result of the RFA was first evaluated by the same method (CT or MRI) of the preprocedural evaluation 1 month after the procedure; in case of complete ablation (CA), follow-up continued with alternating contrast-enhanced US and CT or MRI every 6 months, up to a possible suspicion of local recurrence (LR). In case of partial ablation (PA) or LR, the best treatment option was evaluated on a case-by-case basis, from a new RFA session up to a salvage nephrectomy.
The patients were followed without time limits, if not death (analyzing if related or not to the RM). The possible local or clinical evolutions were as follows: no local residual disease after 1 or 2 sessions (CA), treatment failure (nonretreated PA with LR), death, related or unrelated to the RM; or survival, with or without disease.
On the basis of these parameters, the outcomes have been: technique effectiveness after treatment cycle and maintained during follow-up and survival, including: cumulative, overall survival (OS), cancer-specific survival (CSS) of the 125 RCC patients or of only the 79 newly diagnosed RCC, and disease-free survival (DFS) of the 88 patients with any newly diagnosed RM (79 RCC and 9 benign entities).
As potential predictors, the following were analyzed for technique effectiveness: solitary kidney, side, size and thresholds of 3 and 4 cm, guidance, location and development of RM, approach, previous PN, and treatment of multiple RM in the same session.
Predictors for survival were considered: solitary kidney, histological type, development of the RM, and previous malignancies. When this was limited to the 79 patients with newly diagnosed RCC, the thresholds of 3 cm and T1a were evaluated for OS and CSS; when limited to the 88 with any newly diagnosed RM, the 3- and 4-cm threshold for DFS were evaluated.
Statistical Analysis
For statistical analysis, the SPSS Statistics computer program (IBM, Armonk, NY) was used to evaluate the correlations between the variables mentioned above as well as complications and technique effectiveness, via comparison between means (unpaired t test, two-sided), univariate logistic regression modeling (Fisher’s exact test), and multivariate analysis; and to calculate the survival curves (OS, CSS, and DFS) via the Kaplan–Meier method, also stratified by the aforesaid predictors. Significance was assumed at p < 0.05.
Results
Technical Success
Technical success was achieved in all cases.
Adverse Events
The periprocedural mortality was zero.
AE occurred in 17 RM (8.4 %), including 4 MC (2 %), all before the systematic use of adjunctive techniques. The MC were as follows: a renal bleeding treated with embolization; a urinary leak from renal pelvis caused by a thermal injury, followed by a peritoneal urinoma, treated with percutaneous drainage, nephrostomy, and ureteral stenting; a psoas abscess adjacent to the site of the RFA, successfully drained percutaneously; and a seeding in the abdominal wall.
The correlation between AE or MC and the possible predictors has shown exophytic development (p = 0.0886; odds ratio [OR] 3.760; 95 % confidence interval [CI] 1.015–13.933) and smaller diameter (p = 0.0238; OR 0.934; 95 % CI 0.882–0.989) as protective against AE by both univariate and multivariate analysis; the average diameter of the RM with AE was 35 vs. 29 mm of those without AE, with a threshold of 3 cm (p = 0.0595) approaching significance. No other factors showed prognostic value.
Technique Effectiveness
CA was achieved at the imaging control in 168 RM (83 %) after the first RFA, but when we considered a second session as a part of the course of treatment in 16 cases (8 %), another 8 CA were obtained (87 %).
During follow-up (range 1–109 months, mean 39 months), 4 LR occurred, with maintained CA in 172 RM (85 %) (Table 2). The LR underwent new RFA in 3 cases and radical nephrectomy in 1. Two PA underwent nephrectomy (salvage nephrectomy rate 3 of 203, 1.5 %).
When considering size, the CA was obtained in 160 of 180 RM up to 4 cm (89 %) in 123 of 132 of those up to 3 cm (93 %).
As predictors, size showed a high significance in multivariate analysis for technique effectiveness (OR 0.905; 95 % CI 0.862–0.949), with an average diameter of 28 mm for CA and 38 mm for PA (p < 0.0001). The thresholds of 30 mm (p < 0.0001) and 40 mm (p = 0.0002) were both highly significant for the chance of getting a CA.
Guidance, approach, location, and exophytic development did not reach prognostic values for CA. On the contrary, central development resulted a predictor for treatment failure (p = 0.0038; OR 12.141; 95 % CI 1.902–77.515). Finally, as the sole multivariate analysis, previous PN seemed to be favorable for effectiveness (OR 17.200; 95 % CI 2.131–138.846) and treatment of multiple RM in the same session unfavorable (OR 4.837; 95 % CI 1.583–14.782).
Outcome
Neither impairment of renal function nor metastatic progressions after RFA were found.
Twenty-five of 125 (20 %) patients with RCC died during follow-up, although only 7 died of renal cancer disease (5.5 %). The cumulative survival of all 137 patients at 1, 3, 5, and 10 years was 97, 84, 75, and 63 %. The OS of 125 patients with RCC at 1, 3, and 5 years after treatment was 91, 83, and 73 %, and the CSS was 99, 96, and 91 %. The OS and CSS in the subgroup of 79 newly diagnosed RCC were 93, 83, and 77 %, and 100, 98, and 98 %, respectively (Fig. 2). The 1-, 3-, and 5-year DFS for the 88 patients treated with any newly diagnosed RM was 90, 80, and 75 %.
When we stratified the survival curves of all 137 or of the 125 RCC patients by the variables listed above, no statistically significant differences were obtained.
Considering separately the 79 patients with newly diagnosed RCC, the thresholds of 30 and 40 mm were significant for 3- and 5-year survival (96 and 84, and 87 and 83 %), with p = 0.026 in the first case and p = 0.003 in the second (T1a vs. >T1a). The CSS of these 79 RCC patients at 5 years were 100 % for both ≤30 mm and T1a (significantly higher than stadiums >T1a, p = 0.001) (Fig. 3).
The DFS curves of the 88 patients with any first diagnosis RM at 1, 3, and 5 years were 100, 92, and 86 % for the RM up to 3 cm and 94, 86, and 83 % for those up to 4 cm, with statistical advantage over patients with RM larger than 3 or 4 cm (p < 0.001 and p = 0.001) (Fig. 4) (Table 3).
Discussion
To date, surgical removal remains the standard of care for small renal tumors, and nephron sparing surgery achieves equivalent oncologic outcomes and better preservation of renal function compared with radical nephrectomy [3]. The observation that a significant proportion of small RM are benign tumors or low-grade RCCs with relatively indolent clinical behavior has led to less invasive treatment options for selected patients.
Several publications on RFA have provided data used in the radiological, urological, and oncological guidelines on the management of RM [8–11]. In particular, the 2009 American Urological Association (AUA) guidelines limit the indications of thermal ablations to patients with major comorbidities, increased surgical risk, and clinical T1a, but do not consider them a standard therapy. The 2010 European Association of Urology (EAU) guidelines increase the value of the recommendation (grade A), indicating that patients with small tumors and/or significant comorbidity who are unfit for surgery should be considered for an ablative approach. The 2012 European Association of Medical Oncology (ESMO) guidelines recommend the ablative therapies as an alternative approach in elderly patients with small cortical tumors (≤3 cm), hereditary RCC, and multiple bilateral tumors, with level III and grade C. The limit of 3 cm is based on the strong correlation proved by Best et al. [12] with the long-term outcomes of RFA. According to these results, RFA provides excellent and durable outcomes in tumors smaller than 3 cm, while approximately 20 % of those larger than 3 cm recurred; however, most recurrences were recognized as local and successfully treated with another ablation session. Therefore, we also considered in the assessment of the technique effectiveness a second RFA as part of the course of treatment. Moreover, the CA rate rose from 87 to 93 % when we considered only tumors up to 3 cm, but the thresholds of 4 cm were also statistically highly significant. These findings are in agreement with other long-term local results in the literature [13].
Another reported limit of RFA is the central location, which has been proved to be a predictor of treatment failure also in this series; however, in our subsequent experience, we observed an increase of CA in central tumors, currently equal to that of the noncentral ones. Our impression is that expertise may sometimes decisive in local success and in surgery [14].
Regarding the oncological outcomes, some large series concerning long-term survival have been published. Several authors agree that selection of patients on the basis of tumor characteristics is essential to provide for the long-term efficacy of RFA. For example, Psutka et al. [15], stratifying T1a versus T1b disease, obtained statistically significant differences between both CSS and DFS curves. In our series, considering only the newly diagnosed RCC, we obtained very different OS considering the thresholds of 3 and 4 cm. In addition, cleaning out selection bias from this group (age and comorbidity, the major factors influencing the choice of RFA vs. surgery), i.e., considering CSS, 5-year rate was 100 % for both ≤ 3 cm and T1a RCC, equal to that of Psutka et al. [15] and just higher than that of Olweny et al. [16]. This latter series, which compared RFA with PN in patients with solitary T1a RCC, showed similar long-term oncologic outcomes to nephron-sparing surgery.
RFA has proven benefits in comparison with surgery. Thanks to adjunctive techniques, our data regarding safety align with those of several published series (MC range 0–8.3 %) [13, 17–20] and compare favorably with PN (complication rates up to 20 % [21]).
Another advantage over PN is the better maintenance of renal function, even in cases of preexisting chronic disease [22–25].
The last advantage is the cost containment. In the analysis of Castle et al. [18], costs are lower than PN, and the authors point out that as the oncologic outcomes and safety of these procedures become comparable for cT1a RCC, cost containment will play a larger role in the health care policy setting.
These results lead us to two final considerations. First, RFA may be indicated for RCC up to 3 cm as a first-line treatment for nonelderly patients without comorbidities [3]. Second is a possible answer to the question of Salagierski et al. [26]: “Are small tumors the radiologist’s lesions?” The answer will only be provided by randomized clinical trials.
References
McGovern FJ, Wood BJ, Goldberg SN, Mueller PR (1999) Radio frequency ablation of renal cell carcinoma via image guided needle electrodes. J Urol 161:599–600
Clark TW, Millward SF, Gervais DA, Technology Assessment Committee of the Society of Interventional Radiology et al (2009) Reporting standards for percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol 20(7 suppl):S409–S416
Volpe A, Cadeddu JA, Cestari A et al (2011) Contemporary management of small renal masses. Eur Urol 60:501–515
Gervais DA, McGovern FJ, Arellano RS et al (2003) Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 226:417–424
Silverman SG, Gan YU, Mortele KJ et al (2006) Renal masses in the adult patient: the role of percutaneous biopsy. Radiology 240:6–22
Millet I, Doyon FC, Hoa D (2011) Characterization of small solid renal lesions: can benign and malignant tumors be differentiated with CT? AJR Am J Roentgenol 197:887–896
Patel AR, Lee BH, Campbell SC et al (2011) Bilateral synchronous sporadic renal tumors: pathologic concordance and clinical implications. Urology 78:1095–1099
Veltri A, Grosso M, Castagneri F et al (2009) Radiofrequency thermal ablation of small tumors in transplanted kidneys: an evolving nephron-sparing option. J Vasc Interv Radiol 20:674–679
American Urological Association (AUA) (2009) Guideline for management of the clinical stage 1 renal mass. http://www.auanet.org/common/pdf/education/clinical-guidance/Renal-Mass.pdf
Ljungberg B, Cowanb NC, Hanbury DC et al (2010) EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58:398–406
Escudier B, Eisen T, Porta C (2012) Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(suppl 7):vii65–vii71
Best SL, Park SK, Yaacoub RF et al (2012) Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol 187:1183–1189
Zagoria RJ, Pettus JA, Rogers M et al (2011) Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology 77:1393–1399
Nadu A, Kleinmann N, Laufer M et al (2009) Laparoscopic partial nephrectomy for central tumors: analysis of perioperative outcomes and complications. J Urol 181:42–47
Psutka SP, Feldman AS, McDougal WS et al (2013) Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 63:486–492
Olweny EO, Park SK, Tan YK et al (2012) Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 61:1156–1161
Lian H, Guo H, Zhang G et al (2012) Single-center comparison of complications in laparoscopic and percutaneous radiofrequency ablation with ultrasound guidance for renal tumors. Urology 80:119–125
Castle SM, Gorbatiy V, Ekwenna O et al (2011) Laparoscopic and image-guided radiofrequency ablation of renal tumors: patient selection and outcomes. Curr Urol Rep 12:100–106
Atwell TD, Schmit GD, Boorjian SA et al (2013) Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol 200:461–466
Davis K, Kielar A, Jafari K (2010) Effectiveness of ultrasound-guided radiofrequency ablation in the treatment of 36 renal cell carcinoma tumours compared with published results of using computed tomography guidance. Can Assoc Radiol J 63(3 suppl):S23–S32
Lowrance WT, Yee DS, Savage C et al (2010) Complications after radical and partial nephrectomy as a function of age. J Urol 183:1725–1730
Wehrenberg-Klee E, Clark TW, Malkowicz SB (2012) Impact on renal function of percutaneous thermal ablation of renal masses in patients with preexisting chronic kidney disease. J Vasc Interv Radiol 23:41–45
Turna B, Kaouk JH, Frota R et al (2009) Minimally invasive nephron sparing management for renal tumors in solitary kidneys. J Urol 182:2150–2157
Raman JD, Raj GV, Lucas SM et al (2010) Renal functional outcomes for tumours in a solitary kidney managed by ablative or extirpative techniques. BJU Int 105:496–500
Han JS, Huang WC (2011) Impact of kidney cancer surgery on oncologic and kidney functional outcomes. Am J Kidney Dis 58:846–854
Salagierski M, Akdogan B, Brookman-May S (2013) What is the contemporary role of radiofrequency ablation in the management of small renal masses? Are small lesions the radiologist’s tumors? Eur Urol 63:493–495
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veltri, A., Gazzera, C., Busso, M. et al. T1a as the Sole Selection Criterion for RFA of Renal Masses: Randomized Controlled Trials versus Surgery Should Not Be Postponed. Cardiovasc Intervent Radiol 37, 1292–1298 (2014). https://doi.org/10.1007/s00270-013-0812-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0812-y