Abstract
Purpose
To retrospectively analyze efficacy as measured by volume gain of future remnant liver (FRL) after right portal vein embolization (PVE) using particles only versus particles and additional central plug and/or coil (CP/C) embolization.
Methods
All patients who underwent PVE between July 2011 and December 2012 were retrospectively analyzed. Right PVE was performed either with particle-only (PO) embolization or additional CP/C embolization. All enrolled patients underwent computed tomography or magnetic resonance imaging before PVE and surgery. The images were used for volumetry of the FRL.
Results
Of 75 patients, 40 had PO and 35 CP/C embolization. Age, sex, and tumor entities did not differ significantly between the two groups. Tumor entities included cholangiocarcinoma (n = 52), metastasis from colorectal cancer (n = 14), hepatocellular carcinoma (n = 2), and others (n = 7). Time from PVE to preoperative imaging was similar in both groups. FRL volume before PVE was 329 ± 121 ml in the PO group and 333 ± 135 ml in the CP/C group, and 419 ± 135 ml and 492 ± 165 ml before operation. The average percentage volume gain was significantly higher in the CP/C group than in the PO group, with 53.3 ± 34.5 % versus 30.9 ± 28.8 % (p = 0.002).
Conclusion
Right PVE with additional CP/C embolization leads to a significantly higher gain in FRL volume than embolization with particles alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few decades, technical advances in hepatobiliary surgery have improved perioperative outcome in patients undergoing major hepatic resection [1]. One major development is extensive right liver resection for hilar cholangiocarcinoma and other primary and secondary malignancies in critical localization, affecting the right liver, including segment IV. This strategy allows radical tumor removal and improved recurrence-free and overall survival [2–4]. Postoperative outcome depends—among other things—on the remaining liver volume or future remnant liver volume (FRLV) as morbidity increases when the FRLV in noncirrhotic patients is less than approximately 25 %, and morbidity and mortality increase when the FRLV is less than approximately 40 % of the total liver volume (TLV) in patients with parenchymal disease [5–8]. Moreover, combining measured volume with functional assessment using the innovative LiMAx test allows assessment of the more important future remnant liver (FRL) function for estimation of individual risk and point-of-care treatment [9, 10]. To improve postoperative outcome and to increase the number of resectable tumors, preoperative portal vein embolization (PVE) has become an established strategy in patients in whom the expected FRLV or FRL function is critical. First described in the 1920s, portal vein occlusion leads to a compensatory hypertrophy of the contralateral lobe [11], and therefore embolization of the right portal vein facilitates safe extended right hemihepatectomy after a growth interval of approximately 3–6 weeks [12–19].
Portal vein embolization is performed using a percutaneous transhepatic or intraoperative transileocolic approach. The transileocolic route requires a laparotomy and is therefore used in only approximately 12 % of published cases [15]. Percutaneous transhepatic PVE simply requires ipsilateral or contralateral portal venous puncture; most centers prefer the ipsilateral access in order to avoid vascular complications in the remaining liver lobe [15]. The materials preferably used for PVE are polyvinyl alcohol (PVA) particles, gelatin sponge, fibrin glue, n-butyl cyanoacrylate (NBCA) with lipiodol, polidocanol foam, or combinations of these materials with coils or Amplatzer vascular plugs (AVP). A systematic review by van Lienden et al. [15], shows that gelatin sponge and n-butyl cyanoacrylate are the most widely used materials. They are increasingly combined with central plug and/or coil (CP/C) embolization. One reason is the suspected lower rate of reperfusion leading to a decreased hypertrophy. Another reason is the embolization of segment I, which may sometimes be achieved by central occlusion. However, to our knowledge, there are no clinical studies that compare the effect of different embolization materials on the hypertrophy response [8, 20]. Therefore, the aim of this retrospective study was to analyze the volume gain of the FRL after right PVE in patients who received particle embolization alone compared to patients who received additional CP/C embolization.
Methods
All consecutive patients who underwent PVE between July 2011 and December 2012 were included in our study. All patients were presented to and discussed by an interdisciplinary tumor board, which approved the indication for extended right hemihepatectomy and PVE. The study complied with the Declaration of Helsinki. The study protocol was approved by the institutional review board.
Portal Vein Embolization
Portal vein embolization was performed by using a percutaneous transhepatic access established ipsilaterally via ultrasound- or computed tomography (CT) fluoroscopy-guided direct puncture (21-gauge coaxial needle, 15 cm long; Cook Medical, Bloomington, IN, USA) of a right intrahepatic portal vein (PV) branch. Intraoperative, transileocolic access and consecutive particle-only (PO) embolization was used in one case. A 0.018-inch guide wire was advanced through the needle into the superior mesenteric vein or the splenic vein. The 0.018-inch guide wire was replaced by a stiffer 0.035-inch wire (0.035-inch Safe-T-J-curve; Cook Medical, Bloomington, IN, USA) over a dilatation system (AccuStick II; Boston Scientific, Natick, MA, USA). Over the 0.035-inch guide wire, a tip-marked 4F angiography sheath (23-cm length, opaque tip; Brite-tip sheath; Cordis, Bridgewater, NJ, USA) was introduced in the main PV. Direct portography was performed to visualize PV anatomy. A reverse catheter (Sidewinder 1 tempo; Cordis Corporation, Warren, USA) with a 0.038-inch inner diameter was used to achieve ipsilateral access to the right portal venous system. Branches of right PV were selectively catheterized, and embolization was performed with increasing sizes of PVA particles (250–1,000 μm Contour, Boston Scientific, Natick, MA, USA) until stasis was achieved. In the plug and/or coil group, an AVP type I or II with a diameter of 10–18 mm (St. Jude Medical, St. Paul, MN, USA) and/or large coils (Tornado or Nester Coils 6–10 mm in diameter, Cook Medical, Bloomington, IN, USA) were used to seal the entry of the right main branch or branches. In case of need for clamping of the right main PV during surgery before PV resection, we left the first centimeter after branching of the left PV without plug or coil material. In cases with long enough right PV, a plug was placed. If the right PV was too short for plug placement, coil embolization was performed. Additional coil embolization was necessary in some cases, when no complete thrombosis of the plug was visible after 10 min. In coil-only embolization, the coil embolization could be performed over the 0.038 Sidewider I catheter. In case of plug embolization, the sheath had to be replaced by a 7F or 8F sheath. We decided to take a sheath diameter one French larger than recommended by the manufacturer to allow the parallel placement of a 3F Pigtail catheter with 4 side holes (C3F70P, BALT, Montmorency, France) in the main PV. With this 3F pigtail, the occlusion of the plug could be controlled at any time.
In cases where segment 4 PV branches arise from the left PV, these branches were not selectively embolized because the left PV system was not accessed unless the PV of the right anterior segment originated from the left PV. The success of PVE was controlled by direct portography either of the 4F sheath or over the 3F pigtail catheter. If embolization was still incomplete, larger particles of up to 1,000 μm were administered. During retraction of the sheath, the puncture channel was sealed with 2 ml of fibrinogen (Tissucol Duo; Baxter, Deerfield, IL, USA) to avoid bleeding or bile leakage. Fluoroscopy time and dose–area product were directly taken from the Philips Multidiagnost Eleva (Philips Healthcare, Hamburg, Germany) console.
Volumetry
Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) or contrast-enhanced CT before and approximately 4 weeks after PVE were used for volumetry. MRI volumetry was performed using thin-sliced hepatocyte-phase sequences. For CT volumetry, the venous phase was used.
Liver volume was measured by two clinical radiologists (4 and 16 years of experience) and two liver surgeons in consensus with surgeons using Visage Software 7.1 (Pro Medicus Limited, Richmond, Australia) manually delineating both the right liver lobe, including segments 4a and 4b, and the FRL (generally segments 2 and 3) according to the planned resection line respecting anatomical landmarks.
Body surface area (BSA) was calculated according to Mosteller’s formula. Estimated TLV was calculated from the patient’s BSA using the formula TLV [cm3] = −794.41 + 1,267.28 × BSA [m2] [21].
Anatomic Classification
Portal vein anatomy was classified using direct portography and preoperative cross-sectional images by two radiologists in consensus. The anatomic classification according to Couinaud was modified slightly [22–24]. Normal anatomy with right and left PV and division of right PV in right anterior segment and posterior segment was classified as type I. The other anatomic types are detailed in Fig. 1.
Statistical Analysis
Statistical analysis was performed by PASW Statistics 21 software (IBM, Armonk, NY, USA). To evaluate differences between groups, Student’s t test, Mann–Whitney U-test, Chi square test, or Fisher’s exact test was used according to the type of variable. ANOVA was used to evaluate differences in the anatomic groups. A p-value of <0.05 was considered statistically significant. All quantitative data are expressed as mean ± standard deviation, unless otherwise indicated.
Results
Patient Characteristics
From July 2011 to December 2012, a total of 75 patients were included in our study. The PVE technique was changed from PO to additional CP/C embolization in March 2012. Therefore, 40 patients had PO embolization and 35 additional CP/C embolization (Fig. 2). Age, sex, and tumor entities did not differ significantly between the two groups (Table 1).
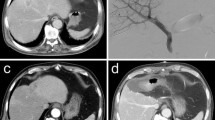
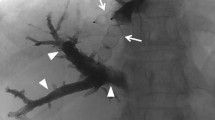
A 66-year-old man with extrahepatic cholangiocarcinoma. Portography shows normal anatomy with right and left portal vein and division of RPV in right anterior and posterior segment (A). After particle embolization (B), a 16-mm type I AVP is placed to seal the entry of the right main branch (C). Residual flow through AVP is embolized with additional coils (D). Final portography over a 3F pigtail catheter in the portal vein shows complete portal devascularization of right liver (E, F)
PV Embolization
The overall technical success rate was 100 %. Most punctures were ultrasound guided (88 % of PO group and 91 % of CP/C group). Fluoroscopy time was significantly higher in the CP/C group at 16.9 ± 6.0 min versus 14.8 ± 8.7 min (p = 0.015) in the PO group. The dose–area product in the CP/C group was higher at 261.9 ± 167.1 Gy * cm2 versus 213.9 ± 166.7 Gy * cm2 in the PO group, although the difference was not statistically significant (p = 0.082) (Table 1). In the CP/C group, a type I AVP with a diameter of 16 mm was used in the majority of cases (79 %). Coils with a diameter of 6–10 mm were used in 64 % of the cases. Details on the embolization materials used in both groups can be found in Tables 2 and 3.
Volumetry
Data on the imaging modalities used before PVE and before resection in the PO and CP/C groups are summarized in Table 1. The time from PVE to preoperative imaging was 29.1 ± 17.9 days in the PO group and 27.7 ± 6.9 in the CP/C group; the slight difference was not statistically significant (p = 0.705).
The ratio between the measured FRLV before embolization and the calculated TLV from BSA did not differ significantly in both groups (p = 0.846).
The average FRL volume of segments 2 and 3 before PVE was 328.9 ± 120.5 ml in the PO group and 332.7 ± 134.9 ml in the CP/C group (p = 0.899). The average FRL volume after PVE before resection was 419.2 ± 134.7 ml in the PO group and 491.7 ± 165.0 ml in the CP/C group, which differed statistically significantly (p = 0.04). The percentage gain in FRL volume in the CP/C group was significantly higher, with 53.3 ± 34.5 % compared to 30.1 ± 28.8 % in the PO group (p = 0.003, Fig. 3). The percentage of FRL volume of TLV before PVE was 18.8 ± 5.3 % for the PO group and 19.1 ± 5.3 % for the CP/C group. The percentage of FRL volume of TLV before resection was 24.7 ± 6.7 % for the PO group and 27.5 ± 5.7 % for the CP/C group. The percentages in both groups before PVE and resection did not differ statistically significantly (p = 0.97 and p = 0.058), but the percentage gain in the ratio of FRL to TLV differed significantly (49.5 ± 24.2 % in the CP/C group versus 31.9 ± 26.6 % in the PO group, p = 0.004; Fig. 4).
PV Anatomy
In the PO group, 29 patients (73 %) had type I, 8 (20 %) type II, and 3 (8 %) type III anatomy. In the CP/C group, 27 patients (77 %) had type I, 4 (11 %) type II, 2 (6 %) type III, and 2 (6 %) type IV anatomy. There was no statistically significant difference in the anatomic types between the two groups (p > 0.05). No statistically significant difference in percentage gain in FRL volume was found according to anatomic type (p > 0.05). Furthermore, no statistically significant differences in fluoroscopy time and dose–area product were found (p > 0.05, Table 4).
Complications and Adverse Events
Four cases of extrahepatic biloma after PVE occurred, 1 (2.5 %) in the PO group and 3 (8.6 %) in the CP/C group, p = 0.334). All were treated with CT-guided percutaneous drainage and did not prohibit the planned resection. Overall, no complications precluding extended hemihepatectomy or PVE-associated deaths occurred.
Surgery
Planned major hepatectomy was conducted in 37 patients (93 %) in the PO group and 33 patients (94 %) in the CP/C group. Reasons for not performing surgery were disease progression in 2 patients and peritoneal carcinomatosis in 1 patient of the PO group and disease progression in 2 patients of the CP/C group. Disease progression in the left liver lobe was not the limiting factor.
Discussion
Today, PVE is the gold standard for inducing hepatic hypertrophy and is considered a reliable and efficient technique [14–16, 19, 22]. Careful patient selection, close collaboration between surgeons and radiologists, individual functional assessment, and better surgical techniques have reduced postoperative mortality of major hepatectomy or hilar en-bloc resection to 2–9 % and have essentially eliminated hepatic insufficiency [4, 10, 25].
Our study has shown that right PVE with particles alone as well as particles and additional CP/C embolization are safe and lead to adequate hypertrophy of the left liver lobe, while the effect of an additional plug and/or coil embolization leads to a significantly higher hypertrophy of the FRL compared to PO embolization. The search for the supreme embolization material combining maximum hypertrophy with minimal adverse events while being effortless to handle is ongoing, and to our knowledge, no direct comparative studies exist.
Van Lienden et al. [15] published a comprehensive review, which—among other aspects—compared the FRL volume increase achieved with different embolic agents, of which NBCA had the most powerful effect. Although the follow-up interval may vary, seven studies including a total of 424 patients reported results on NBCA embolization; all data pooled showed an average percentage FRL volume increase of 49.9 %. We achieved a comparable average percentage FRL volume increase of 53.3 % in our patients. Some studied report an average percentage FRL volume increase of up to 74 %, which may be significantly higher than our results [26]. There are, however, two potential disadvantages of NBCA as an embolic agent for PVE. First, NBCA is more difficult to administer because it bears the risk of nontarget embolization, especially in patients with reduced hepatopetal portal flow. Second, it generates a peribiliary fibrosis, which may render hemihepatectomy more difficult [27, 28]. The advantage of NBCA is that material costs are significant lower compared to particle or particle and plug/coil embolization.
Few clinical studies or case series exist that investigate the use of AVP combined with NBCA or PVA particles as a closure device for PVE; they report average percentage FRL volume increases of 27–68.9 % [20, 29–32]. Several cases of recanalized PV branches have been reported after PVE with particles alone [33, 34]; thus, the combination with AVP and large coils to prevent recanalization is compelling, and although we did not specifically investigate visible recanalization of major portal branches on follow-up imaging, our results show a significantly higher hypertrophy rate, which we explain by the combination of peripheral and central PV occlusion and therefore a minimum of reperfusion. Central plug/coil embolization enables precise release and possible repositioning, thereby minimizing the risk of nontarget embolization. By using AVP, the use of stainless steel coils—which result in significant artefacts on follow-up CT or MRI—can be decreased.
Embolization with an additional plug and/or coils had a higher incidence of biloma after PVE (three in the CP/C group and one in the PO group). This can be explained by the larger sheath (7–8F) that is required to deliver the AVP. Biloma, however, did not interfere with the planned surgical resection and could be treated by CT-guided drainage in all cases.
Our study has several limitations. First, the study design was retrospective; a prospective randomized trial would have better validity. Second, the imaging modalities before PVE and resection differed between MRI and CT and were not the same in all patients. CT and MRI measurements give slightly different, but not significantly different, volumes after manual volumetry, mainly because of breathing and partial volume effects [35, 36]. Finally, because selective embolization of segment IV branches was not performed and this segment is supplied by branches from either the left or right PV or from both, the distribution regarding the actual embolization of segment IV in the two groups may be different.
With increasing indications for PVE, further studies are needed to compare the competing embolic agents NBCA with PVA plus plug/coil embolization regarding the hypertrophy rate and the safety profile in a prospective and randomized setting.
A number of alternative methods to generate FRL volume gain should be mentioned. First, there is the possibility of hepatic artery embolization, which has, however, been shown to be less effective than PVE [12]. Another alternative is intraoperative PV ligation, which also seems to be less effective than PVE and can complicate the definitive resection [37]. In-situ splitting with PV ligation represents an alternate method, but one with a significantly higher mortality and morbidity rate, which has yet to be explored further [38].
In conclusion, right PVE with additional CP/C embolization leads to a significantly higher gain in FRL volume than embolization with particles alone.
References
Agrawal S, Belghiti J (2011) Oncologic resection for malignant tumors of the liver. Ann Surg 253:656–665
Dinant S, Gerhards MF, Rauws EA et al (2006) Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol 13:872–880
Neuhaus P, Jonas S, Bechstein WO et al (1999) Extended resections for hilar cholangiocarcinoma. Ann Surg 230:808–818
Neuhaus P, Thelen A, Jonas S et al (2012) Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol 19:1602–1608
Abdalla EK, Barnett CC, Doherty D et al (2002) Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg 137:675–680
Kubota K, Makuuchi M, Kusaka K et al (1997) Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 26:1176–1181
Shirabe K, Shimada M, Gion T et al (1999) Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 188:304–309
Madoff DC, Abdalla EK, Gupta S et al (2005) Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 16(2 pt 1):215–225
Stockmann M, Lock JF, Riecke B et al (2009) Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg 250:119–125
Stockmann M, Lock JF, Malinowski M et al (2010) The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford) 12:139–146
Rous P, Larimore LD (1920) Relation of the portal blood to liver maintenance: a demonstration of liver atrophy conditional on compensation. J Exp Med 31:609–632
Denecke T, Seehofer D, Steffen IG et al (2011) Arterial versus portal venous embolization for induction of hepatic hypertrophy before extended right hemihepatectomy in hilar cholangiocarcinomas: a prospective randomized study. J Vasc Interv Radiol 22:1254–1262
de Graaf W, van Lienden KP, van den Esschert JW et al (2011) Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg 98:825–8834
Avritscher R, de Baere T, Murthy R et al (2008) Percutaneous transhepatic portal vein embolization: rationale, technique, and outcomes. Semin Interv Radiol 25:132–145
van Lienden KP, van den Esschert JW, de Graaf W et al (2012) Portal vein embolization before liver resection: a systematic review. Cardiovasc Interv Radiol 36:25–34
Madoff DC, Abdalla EK, Vauthey JN (2005) Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol 16:779–790
Abdalla EK, Hicks ME, Vauthey JN (2002) Portal vein embolization: rationale, technique and future prospects. Br J Surg 88:165–175
Liu H, Zhu S (2009) Present status and future perspectives of preoperative portal vein embolization. Am J Surg 197:686–690
May BJ, Talenfeld AD, Madoff DC (2013) Update on portal vein embolization: evidence-based outcomes, controversies, and novel strategies. J Vasc Interv Radiol 24:241–254
Libicher M, Herbrik M, Stippel D et al (2010) Portal vein embolization using the Amplatzer vascular plug II: preliminary results. Rofo 182:501–506
Chun YS, Ribero D, Abdalla EK et al (2008) Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg 12:123–128
Madoff DC, Hicks ME, Vauthey JN et al (2002) Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics 22:1063–1076
Akgul E, Inal M, Soyupak S et al (2002) Portal venous variations. Prevalence with contrast-enhanced helical CT. Acta Radiol 43:315–319
Couinaud C (1957) Le foie. In: Etudes anatomiques et chirurgicales. Masson, Paris, p 71
Vauthey JN, Pawlik TM, Abdalla EK et al (2004) Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg 239:722–732
Guiu B, Bize P, Gunthern D et al (2013) Portal vein embolization before right hepatectomy: improved results using n-butyl-cyanoacrylate compared to microparticles plus coils. Cardiovasc Interv Radiol 36:1306–1312
de Baere T, Roche A, Elias D et al (1996) Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology 24:1386–1391
Imamura H, Shimada R, Kubota M et al (1999) Preoperative portal vein embolization: an audit of 84 patients. Hepatology 29:1099–1105
Bent CL, Low D, Matson MB et al (2009) Portal vein embolization using a nitinol plug (Amplatzer vascular plug) in combination with histoacryl glue and iodinized oil: adequate hypertrophy with a reduced risk of nontarget embolization. Cardiovasc Interv Radiol 32:471–477
Yoo H, Ko GY, Gwon DI et al (2009) Preoperative portal vein embolization using an Amplatzer vascular plug. Eur Radiol 19:1054–1061
Kalenderian AC, Chabrot P, Buc E et al (2011) Preoperative portal vein embolization with Amplatzer(®) vascular plugs (AVP): a review of 17 cases. J Radiol 92:899–908
Ringe KI, Weidemann J, Rosenthal H et al (2007) Transhepatic preoperative portal vein embolization using the Amplatzer vascular plug: report of four cases. Cardiovasc Interv Radiol 30:1245–1247
Covey AM, Tuorto S, Brody LA et al (2005) Safety and efficacy of preoperative portal vein embolization with polyvinyl alcohol in 58 patients with liver metastases. AJR Am J Roentgenol 185:1620–1626
Pavcnik D, Saxon RR, Kubota Y et al (1997) Attempted induction of chronic portal venous hypertension with polyvinyl alcohol particles in swine. J Vasc Interv Radiol 8(1 pt 1):123–128
Karlo C, Reiner CS, Stolzmann P et al (2010) CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol 75:e107–e111
Reiner CS, Karlo C, Petrowsky H et al (2009) Preoperative liver volumetry: how does the slice thickness influence the multidetector computed tomography– and magnetic resonance–liver volume measurements? J Comput Assist Tomogr 33:390–397
Robles R, Marín C, Lopez-Conesa A et al (2012) Comparative study of right portal vein ligation versus embolisation for induction of hypertrophy in two-stage hepatectomy for multiple bilateral colorectal liver metastases. Eur J Surg Oncol 38:586–593
Schnitzbauer AA, Lang SA, Goessmann H et al (2012) Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 255:405–414
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dominik Geisel, Maciej Malinowski, Martin Stockmann and Bernhard Gebauer have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Geisel, D., Malinowski, M., Powerski, MJ. et al. Improved Hypertrophy of Future Remnant Liver after Portal Vein Embolization with Plugs, Coils and Particles. Cardiovasc Intervent Radiol 37, 1251–1258 (2014). https://doi.org/10.1007/s00270-013-0810-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0810-0