Abstract
Purpose
To assess the clinical outcomes of internal iliac artery (IIA) embolization before endovascular aneurysm repair (EVAR).
Methods
Between 2002 and 2011, 88 patients underwent IIA embolization prior to EVAR. Sixty-five patients underwent unilateral and 23 underwent bilateral IIA embolization. A total of 111 IIAs were embolized: 56 were embolized with coils, 41 with Amplatzer plugs, and 14 with a combination of embolic agents. The outcomes were assessed retrospectively by reviewing medical records and follow-up imaging.
Results
IIA embolization was technically successful in 95.7 % of cases. Type 2 endoleak from previously embolized IIAs was seen in 4 cases, and in 1 case this was significant necessitating re-intervention. Buttock claudication was reported in 38 % of cases, whereas new onset erectile dysfunction occurred in 10 % of cases. No severe ischemic complications, such as spinal cord ischaemia or buttock necrosis, were reported. Analysis comparing unilateral versus bilateral embolization, simultaneous versus sequential embolization, and the type of embolic material used showed no statistical significance.
Conclusion
IIA embolization is technically successful and effective in preventing significant type 2 endoleak in the majority of cases. It is a relatively safe procedure without major complications, but the incidence of buttock claudication and erectile dysfunction remain relatively high, and patients should be consented appropriately. There is no significant benefit for adopting a particular embolization technique, but there is a tendency towards reduced pelvic ischaemia with proximal embolization. Four cases of type II endoleak occurring after technically successful IIA embolization supports the school of thought that IIA should be embolized prior to coverage and extension of the distal landing zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The scope of endovascular repair of abdominal aortic aneurysms (AAA) has expanded greatly since its first inception by Parodi et al. [1] in 1991. Improvements in endograft design and increasing experience with endovascular techniques have facilitated the treatment of more morphologically complex aneurysms. Aortoiliac aneurysms lack a suitable common iliac artery (CIA) landing zone and require extension of the iliac limb into the external iliac artery (EIA), and therefore coverage of the internal iliac artery (IIA) origin. There has been continued debate regarding the fate of IIAs, i.e. whether they should be sacrificed by embolization to prevent type II endoleak caused by retrograde perfusion of the IIA.
During the past decade or so, IIA embolization has extended the suitability of endovascular repair of aortoiliac aneurysms from 27 to 43 % of patients [2]. However, concern remains about the sacrifice of IIAs, which may lead to pelvic ischaemia. The majority of complications are minor, such as buttock claudication and sexual dysfunction, but may be more severe, such as buttock necrosis, paraplegia and bowel ischaemia [2–8].
The aim of this single-centre retrospective analysis is to review the immediate and long-term outcomes of unilateral and bilateral IIA embolization prior to EVAR, with respect to morbidity and complications related to pelvic ischaemia. In addition, we assessed whether certain factors—such as unilateral versus bilateral embolization, simultaneous versus sequential embolization, and distal versus proximal embolization—increase the risk of pelvic ischaemia.
Materials and Methods
Patients
During an 8-year period (2002–2011), 88 patients underwent unilateral or bilateral IIA embolization prior to endovascular repair of aortoiliac or iliac aneurysms. The mean age was 79 ± 7.35 years (range 59–92), and only 5 patients were female. Forty-one patients presented with aorto-uni-iliac aneurysms, 34 with aorto-bi-iliac aneurysms, 6 with bilateral CIA aneurysms, 4 with unilateral CIA aneurysms, and 3 with abdominal aortic and IIA aneurysms.
The medical records, pre-procedural imaging, and follow-up imaging of these patients were reviewed retrospectively. In addition, telephone interviews were conducted with all available patients regarding complications related to pelvic ischaemia.
Embolization Technique
Informed consent was obtained from all patients. Routine computed tomography (CT) was performed to plan the endovascular repair and to confirm the necessity for iliac limb extension and coverage of the IIA. In all cases, IIA embolization was performed before stent-graft placement, either at the time of EVAR or as a separate procedure prior to EVAR. CT angiograms were used to review the aortoiliac anatomy, tortuosity of vessels, and IIA diameter. This information was used to decide the optimal approach for successful catheterization and the ideal embolic material.
The choice of ipsilateral versus contralateral femoral access was left to the decision of the operator and was generally dependent on the individual patient’s anatomy. In a proportion of patients, both the contralateral and ipsilateral access routes were used if the initial approach to the internal iliac artery was unsuccessful.
A total of 111 IIAs were embolized in the 88 patients treated. Unilateral embolization was performed in 65 patients and bilateral embolization in 23 patients. Of those requiring bilateral embolization, 18 patients underwent sequential embolization, and 5 patients underwent simultaneous embolization during the same procedure (Fig. 1).
The majority of IIAs were embolized with either coils (n = 56) or vascular plugs (n = 42) and the remainder with a mixture of embolic materials, such as coils with gelfoam (n = 11) and vascular plug with coils (n = 2). The choice of embolic material was at the discretion of the operator. However, in general, coils were the preferred embolic agent of choice early in the series. With the introduction of the Amplatzer plug on to the market, this device gradually became the most commonly used embolic agent for this indication.
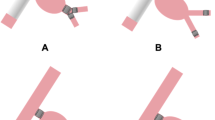
The types of coils used were a combination of MReye (William Cook Europe, Bjaeverskov, Denmark), Nester (William Cook Europe), Spiral coils (Balt Extrusion, Montmorency, France), and platinum microcoils (William Cook Europe). Both type I and II Amplatzer vascular plugs (AGA Medical Corporation, MN, USA) were used to embolize the main trunk of the IIA in our series (Fig. 2). However, the type 2 plug was more commonly used than the type 1 plug later in the series. In one patient with an internal iliac artery aneurysm, type 4 Amplatzer plugs were used to occlude the branches of the internal iliac artery.
Proximal internal iliac artery embolization using a vascular plug. A Right internal iliac artery catheterization. B Sheath advancement and placement of Amplatzer II plug in main trunk of internal iliac artery. C Occlusion of antegrade flow of internal iliac artery. D Subsequent extension of right iliac limb to ipsilateral external iliac artery
As a general rule, our aim was to place the embolic material in the proximal portion of the IIA, thus allowing future peripheral collateralization from the IIA branches to decrease the risk of pelvic ischaemia. In the three cases in which concurrent AAA and IIA aneurysms were present, the branches of IIAs were intentionally occluded to minimize the risk of persistent type 2 endoleak (Fig. 3).
Coils and Amplatzer plug diameters were selected according to the IIA diameter and oversized by ~20–30 %. Coils were placed through standard 5–6 Fr sheaths after ipsilateral or contralateral catheterization with a 5F catheter. Only in cases where stable selective catheterization of the IIA could not be achieved, were microcatheter and microcoils used. Amplatzer plugs were mostly deployed from the contralateral approach using 6–8 Fr, long, braided sheaths across the aortic bifurcation and into the IIA origin. In most cases, one Amplatzer plug was sufficient to occlude the main trunk of the IIA. However, two cases required additional coils for successful vessel occlusion.
Technical success was defined as the total or subtotal cessation of antegrade flow in the IIA trunk or its divisions depending on the site of embolization. The embolization procedure was terminated at the discretion of the operator when total occlusion was achieved or the remaining flow was considered insignificant enough to result in imminent total occlusion. Therefore, minor residual flow in the IIA after embolization was tolerated.
Follow-Up
Routine clinical and imaging follow-up was performed at 1, 3, 6, and 12 months after EVAR and annually thereafter according to our institutional protocol. Early in our experience, CT and plain abdominal radiographs (AXR) were the routine imaging modalities for follow-up after EVAR. More recently, color duplex ultrasound has replaced CT, with CT now reserved for abnormalities found on either ultrasound or AXR. At the clinic visit, each patient was assessed for any claudication symptoms and existing erectile dysfunction before the procedure and any new onset symptoms of pelvic ischaemia were documented on future clinic visits.
In addition, at the time of the data analysis, surviving patients were contacted by telephone by the first and third authors of this manuscript. Patients who could be contacted successfully were asked specifically regarding symptoms related to pelvic ischaemia, particularly buttock claudication and erectile dysfunction.
Statistical Analysis
The incidence of buttock claudication and erectile dysfunction were compared between different patient subgroups using a two-tailed Fisher’s exact test. A p-value of <0.05 was considered to be statistically significant. Comparisons were made between patients who underwent unilateral versus bilateral IIA embolization, proximal versus distal embolization, and if bilateral embolization was performed, between those who underwent sequential versus simultaneous embolization.
Results
Technical Success
Embolization of the IIA was technically successful in 95.7 % of cases (111 of 116 embolization procedures). This was confirmed angiographically during subsequent EVAR. The five patients with failed IIA embolization were patients who were originally scheduled for bilateral IIA embolization; these patients had successful contralateral (i.e. unilateral) IIA embolization procedures. No type II endoleak occurred from the IIAs that could not be embolized. Coverage of the IIA origin during EVAR with limb extension into the EIA resulted in subsequent thrombosis of the main IIA trunk and was confirmed on follow-up CT. No significant IIA stenosis was seen in these cases prior to EVAR.
There were three cases of intra-procedural coil migration into the EIA, but these coils were successfully retrieved without any clinical sequelae. No immediate complications, such as puncture site haematoma, arterial injury, or non-target embolization, were reported.
Type II Endoleak
On imaging follow-up, 4 of 111 embolized IIAs (3.6 %) showed type II endoleak between the first week and second month after EVAR. Two had been embolized with coils and the other two with Amplatzer plug. Only one of these endoleaks resulted in an increase in aneurysm sac diameter. This patient had undergone coil embolization of left IIA branches prior to endovascular repair of AAA and concomitant left IIA aneurysm. A type 2 endoleak was seen arising from recanalized IIA branches on routine follow-up CT at 2 months. The IIA aneurysm was then noted to have increased in size at 6 months. Therefore, a secondary embolization was performed by way of a percutaneous approach, which was successful in arresting sac growth.
Pelvic Ischaemia
It was possible to contact 47 of our original 88 patients for a telephone interview regarding complications, particularly buttock claudication and erectile dysfunction. Eighteen patients (38 %) reported a history of buttock claudication after IIA embolization. Claudication distance was <50 m in 4 patients, 50–100 m in 3 patients, 100–500 m in 8 patients, and >500 m in 3 patients. Symptoms were transient in 6 patients lasting 3–12 months, and ongoing (>1 year) in 12 patients lasting between 15 months to 7 years. Eight of those with ongoing symptoms reported that the claudication was lifestyle-limiting due to restrictions on their mobility. Of the patients who experienced buttock claudication, 12 (67 %) had undergone unilateral IIA embolization, and 6 (33 %) had undergone bilateral embolization. Of the 6 bilateral embolizations, 1 patient underwent simultaneous embolization, whereas the remaining 5 patients underwent sequential embolization. Proximal occlusion with a vascular plug or coils was performed in 5 cases (28 2%), whereas distal embolization with coils was performed in 13 cases (72 %). There was no statistical significance between the incidence of buttock claudication in unilateral versus bilateral embolization (p = 0.150), simultaneous versus sequential embolization (p = 1.0), and proximal versus distal embolization (p = 0.137). In addition, there was no correlation between the duration of symptoms and embolization technique.
New onset erectile dysfunction was reported by 4 of 42 interviewed male patients (10 %) aged between 65–79 years. Three additional patients reported continued symptoms that predated the embolization procedure. All four patients who reported new onset erectile dysfunction underwent unilateral embolization. The contralateral IIAs were patent in these patients although significant atherosclerotic disease was seen. Two of the 4 (50 %) patients were embolized proximally, and the remaining 2 patients were embolized distally. There was no statistically significant difference between unilateral versus bilateral embolization (p = 0.561) and proximal versus distal embolization (p = 1.0). Other rarer but more serious pelvic ischaemia complications, such as spinal ischaemia, ischaemic colitis, and buttock necrosis, were not reported in our series.
Unilateral Versus Bilateral Embolization
Of the 47 patients who could be interviewed regarding pelvic ischaemic complications, either symptoms of buttock claudication or erectile dysfunction were reported by 20 patients. Symptoms occurred in 14 of 37 (38 %) patients who underwent unilateral embolization and in 6 of 10 (60 %) patients who underwent bilateral embolization (Table 1). There was no statistically significant difference with respect to clinical symptoms between unilateral versus bilateral embolization (p = 0.286). Only 2 patients experienced both symptoms of buttock claudication and erectile dysfunction: One patient underwent unilateral embolization, and the other patient underwent bilateral embolization.
Proximal Versus Distal Embolization
Of the 47 patients who could be interviewed, pelvic ischaemic symptoms were reported in 6 of 20 (30 %) of patients who underwent proximal embolization of the main trunk of the IIA. In comparison, 14 of 27 (52 %) of those who underwent a more distal embolization developed ischaemic complications (Table 2). Again, there was no statistical significant difference between the 2 groups (p = 0.152).
Simultaneous Versus Sequential Embolization
Of the 47 patients who could be interviewed for pelvic ischaemic symptoms, only 10 had undergone bilateral embolization. Buttock claudication or new onset erectile dysfunction were seen in 1 of 2 (50 %) patients who underwent simultaneous bilateral embolization and in 6 of 8 (75 %) of those who underwent sequential bilateral embolization. There was no statistical significance between the incidence of pelvic ischaemia in simultaneous versus sequential bilateral embolization (p = 1.0).
Discussion
IIA embolization was introduced as a treatment option to prevent type 2 endoleaks from the IIA when the stent-graft limbs need to be extended into the EIA [2, 9, 10]. However, there is concern regarding the sacrifice of the IIAs by embolization as many operators believe this exposes the patient to the risk of pelvic ischaemic complications. In our institution, until the introduction of iliac branched devices, we have routinely performed IIA embolization prior to EVAR when extension of a limb to the EIA has been considered to be necessary because of an aneurysmal or short CIA. As a result, this cohort of patients represents one of the largest reported series of those undergoing IIA embolization before EVAR.
Although the anatomy of aortoiliac aneurysms can be technically challenging to navigate during IIA embolization, the procedure is performed safely with high technical success rates approaching 100 % [2, 11, 12]. The standard coil embolization technique has been described as both safe and effective [2, 13–15]. Coil migration has been described, but all misplaced coils have been retrieved without clinical consequences [2, 11]. More serious complications, such as IIA dissection and non-target organ embolization, are rare [2, 11, 16]. Similarly, our series reports a technical success rate of 96 % with no significant immediate complications and successful retrieval of the few coils that migrated.
It is interesting to note that the four unsuccessful cases did not result in type 2 endoleak. Coverage of the IIA origin during EVAR resulted in thrombosis of the main IIA trunk, raising the question of the necessity of IIA embolization prior to endograft extension into the EIA. Several recent reports have suggested that the distal landing zone can be safely extended into the EIA without the need for IIA embolization [17–20]. These studies have reported no type II endoleaks related to the IIA and a lower buttock claudication rate of 27 % compared with 45 % of those treated with coil embolization [18]. An earlier series from our institution evaluated a group of patients in whom intended coil embolization was unsuccessful before EVAR [17]. The investigators reported that all patients who underwent endograft coverage of the IIA subsequently thrombosed the IIA trunk and that failure to embolize the IIA should not preclude patients from treatment [17].
In contrast, our current series supports the necessity of IIA embolization before coverage and limb extension. Evidence suggests that type II endoleaks that arise from a previously embolized IIA are rare [16, 21], and when encountered, are typically self-limiting and do not require treatment [17]. However, there were four cases of type II endoleak from previously embolized IIAs in our study group, all of which were deemed technically successful at the time of the occlusion. One of these cases required further embolization because it resulted in an enlarging aneurysm sac. This suggests that the incidence of clinically significant type II endoleak may be significantly greater without previous IIA embolization.
The available literature regarding the severity and frequency of pelvic ischaemic complications after IIA embolization remains heterogeneous and inconclusive [22, 23]. Previous studies have not analyzed outcomes with respect to the variability of embolization techniques (e.g. unilateral versus bilateral, proximal versus distal, sequential versus simultaneous, and type of embolic material), and have shown varying complication rates ranging between zero and 80 % [2, 13, 15, 24–26]. A recent systematic review of the available literature reported an overall incidence of 28 % [23]. Table 3 lists the reported incidence of buttock claudication and erectile dysfunction from recent studies.
The incidence of buttock claudication immediately after embolization has been reported to be between 16 [22] and 55 % [23] with a decreasing incidence during the follow-up period and only a small percentage of patients experiencing persistent symptoms. For example, an immediate buttock claudication rate of 16 % decreased to 2 % after 12 months in one study [25], and buttock/thigh claudication in 30 % of patients soon after embolization resolved in the majority after 1 year in another study [11]. Our series reported an initial buttock claudication rate of 38 %, which lasted >12 months in 26 % and was lifestyle-limiting in 17 %. There were no cases of delayed onset buttock claudication in our patients.
Sexual dysfunction after IIA embolization has an overall reported incidence of 17 % [23]. However, a significant proportion of male patients who are referred for IIA embolization will have pre-existing sexual dysfunction before the embolization procedure, in as high as 53 % in the series reported by Razavi et al. [26]. Similarly, our series showed that pre-existing erectile problems were present in 7 % of patients, and new onset erectile dysfunction occurred in 10 % of patients. Severe pelvic ischaemic symptoms, such as paraplegia, colonic ischaemia, and buttock necrosis, have been described but rarely occur [11, 25, 27].
There is a general consensus in the literature that bilateral embolization increases the risk of pelvic ischaemia. Moreover, presence of >70 % stenosis in the contralateral IIA, occlusion of more than three IIA branches, and absence of ascending femoral branches have been identified as risk factors for post embolization pelvic ischaemia [9]. However, a recent systematic review found no significant difference in the incidence of buttock claudication and erectile dysfunction between unilateral or bilateral occlusions [23]. An earlier report from our institution also found severe complications after bilateral IIA embolization to be uncommon [11]. Similarly, our current series also supports the notion that bilateral embolization is not associated with significantly worse pelvic ischaemia than unilateral IIA embolization.
In patients who have bilateral CIA aneurysms, it may theoretically seem beneficial to perform staged IIA embolization to allow sufficient time for collateral vessels to develop. However, a study measuring pressures in the IIA after clamping the contralateral IIA and ipsilateral EIA suggested that branches of the ipsilateral EIA provide a more significant collateral pathway than the contralateral IIA [28]. Accordingly, an earlier series from our institution showed no significant difference in the rate of severe ischaemic complications in patients who underwent sequential versus simultaneous embolization [11]. The results of our current series are also in line with these findings.
Proximal occlusion of the IIA at the main trunk is thought to enable development of a collateral circulation and retroperfusion of the pelvic branches [29]. Therefore, it is considered better practice to embolize the main trunk of the IIA rather than distal to the IIA bifurcation [11, 21, 30, 31]. This is supported by an earlier report from our institution, which showed a significant difference in the rate of ischaemic complications based on the level of IIA embolization [11], with 55 % of patients treated using distal embolization experiencing ischaemic complications compared with only 16 % of patients after more proximal embolization [11]. Although our current series showed a slight tendency for distal embolization to cause an increased incidence of pelvic ischaemia, statistical significance could not be demonstrated.
Iliac branch graft devices (IBDs) are a relatively recent development consisting of an iliac extension limb with a side branch for implantation into the IIA, thereby preserving flow in the IIA. These devices are being used more frequently in some centers in preference to embolization of the IIA. However, certain morphological criteria must be met for successful deployment of these devices, which may exclude a significant proportion of aortoiliac aneurysms. A retrospective study comparing IBDs versus IIA embolization showed similar immediate technical success rates (94 and 93 %, respectively) [32]. Re-intervention rates were lower in the IIA embolization group at 9.5 % compared with 16 % in the IBD group (p = 0.2). Interestingly, this study did not demonstrate a statistically significant difference between the two groups with respect to the rate of pelvic ischaemia.
There are limitations to this study. Thirty-one of the 88 patients in our study group could not be contacted for an interview regarding their pelvic ischaemic symptoms. This dilutes the strength of our results, especially the subgroup analyses, in which the numbers were relatively small.
Conclusion
The results of this study show that embolization of the IIA is effective in preventing type 2 endoleaks in the majority of patients. The procedure is considered safe without any major complications. However, the incidence of buttock claudication and erectile dysfunction remain relatively high, and patients should be informed as such before the procedure. There is no significant benefit for adopting a particular embolization technique, i.e. unilateral versus bilateral, sequential versus simultaneous, or proximal versus distal embolization. However, there is a tendency towards reduced pelvic ischaemia with proximal versus distal embolization. There were four cases of type II endoleak following technically successful IIA embolization, one resulting in an increase in aneurysm sac diameter and requiring re-intervention. This supports the school of thought that IIA should be embolized prior to coverage and extension of the distal landing zone.
References
Parodi JC, Palmaz JC, Barone HD (1991) Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 5:491–499
Schoder M, Zaunbauer L, Hölzenbein T et al (2001) Internal iliac artery embolization before endovascular repair of abdominal aortic aneurysms: frequency, efficacy, and clinical results. AJR Am J Roentgenol 177:599–605
Andriole GL, Sugarbaker PH (1985) Perineal and bladder necrosis following bilateral internal iliac artery ligation. Report of a case. Dis Colon Rectum 28:183–184
Senapati A, Browse NL (1990) Gluteal necrosis and paraplegia following postoperative bilateral internal iliac artery occlusion. J Cardiovasc Surg 31:194–196
Gloviczki P, Cross SA, Stanson AW et al (1991) Ischemic injury to the spinal cord or lumbosacral plexus after aorto-iliac reconstruction. Am J Surg 162:131–136
Cikrit DF, O’Donnell DM, Dalsing MC et al (1991) Clinical implications of combined hypogastric and profunda femoral artery occlusion. Am J Surg 162:137–140
Paty PK, Shah DM, Chang BB et al (1994) Pelvic ischemia following aortoiliac reconstruction. Ann Vasc Surg 8:204–206
Shin RK, Stecker MM, Imbsei SG (2001) Peripheral nerve ischaemia after internal iliac artery ligation. J Neurol Neruosurg Psychiatry 70:411–412
Yano OJ, Morrissey N, Eisen L et al (2001) Intentional internal iliac artery occlusion to facilitate endovascular repair of aortoiliac aneurysms. J Vasc Surg 34:204–211
Bayly PJ, Matthews JN, Dobson PM et al (2001) In-hospital mortality from abdominal aortic surgery in Great Britain and Ireland: Vascular Anaesthesia Society audit. Br J Surg 88:687–692
Bratby MJ, Munneke GM, Belli AM et al (2008) How safe is bilateral internal iliac artery embolization prior to EVAR? Cardiovasc Intervent Radiol 31:246–253
Maleux G, Willems E, Vaninbroukx J et al (2010) Outcome of proximal internal iliac artery coil embolization prior to stent-graft extension in patients previously treated by endovascular aortic repair. J Vasc Interv Radiol 21:990–994
Wolpert LM, Dittrich KP, Hallisey MJ et al (2001) Hypogastric artery embolization in endovascular abdominal aortic aneurysm repair. J Vasc Surg 33:1193–1198
Karch LA, Hodgson KL, Mattos MA et al (2000) Adverse consequences of internal iliac artery occlusion during endovascular repair of abdominal aortic aneurysms. J Vasc Surg 32:676–683
Criado FJ, Wilson EP, Velazquez OC et al (2000) Safety of coil embolization of the internal iliac artery in endovascular grafting of abdominal aortic aneurysms. J Vasc Surg 32:684–688
Pellerin O, Caruba T, Kandounakis Y et al (2008) Embolization of the internal iliac artery: cost-effectiveness of two different techniques. Cardiovasc Intervent Radiol 31:1088–1093
Bharwani N, Raja J, Choke E et al (2008) Is internal iliac artery embolization essential prior to endovascular repair of aortoiliac aneurysms? Cardiovasc Intervent Radiol 31:504–508
Wyers MC, Schermerhorn ML, Fillinger MF et al (2002) Internal iliac occlusion without coil embolization during endovascular abdominal aortic aneurysm repair. J Vasc Surg 36:1138–1145
Tefera G, Turnipseed WD, Carr SC et al (2004) Is coil embolization of hypogastric artery necessary during endovascular treatment of aortoiliac aneurysms? Ann Vasc Surg 18:143–146
Mell M, Tefera G, Schwarze M et al (2006) Absence of buttock claudication following stent-graft coverage of the hypogastric artery without coil embolization in endovascular aneurysm repair. J Endovasc Ther 13:415–419
Engelke C, Elford J, Morgan RA et al (2002) Internal iliac artery embolization with bilateral occlusion before endovascular aortoiliac aneurysm repair-clinical outcome of simultaneous and sequential intervention. J Vasc Interv Radiol 13:667–676
Mehta M, Veith FJ, Darling RC et al (2004) Effects of bilateral hypogastric artery interruption during endovascular and open aortoiliac aneurysm repair. J Vasc Surg 40:698–702
Rayt HS, Bown MJ, Lambert KV et al (2008) Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Intervent Radiol 31:728–734
Lee CW, Kaufman JA, Fan CM et al (2000) Clinical outcome of internal iliac artery occlusions during endovascular treatment of aortoiliac aneurysmal diseases. J Vasc Interv Radiol 11:567–571
Mehta M, Veith FJ, Ohki T et al (2001) Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: a relatively innocuous procedure. J Vasc Surg 33(Suppl 2):S27–S32
Razavi MK, DeGroot M, Olcott C 3rd et al (2000) Internal iliac artery embolization in the stent-graft treatment of aortoiliac aneurysms: analysis of outcomes and complications. J Vasc Interv Radiol 11:561–566
Lin PH, Bush RL, Chaikof EL et al (2002) A prospective evaluation of hypogastric artery embolization in endovascular aortoiliac aneurysm repair. J Vasc Surg 36:500–506
Iliopoulos JI, Hermreck AS, Thomas JH et al (1989) Hemodynamics of the hypogastric arterial circulation. J Vasc Surg 9:637–641
Maldonado TS, Ranson ME, Rockman CB et al (2007) Decreased ischemic complications after endovascular aortic aneurysm repair with newer devices. Vasc Endovascular Surg 41:192–199
Cynamon J, Lerer D, Veith FJ et al (2000) Hypogastric artery coil embolization prior to endoluminal repair of aneurysms and fistulas: buttock claudication, a recognized but possibly preventable complication. J Vasc Interv Radiol 11:573–577
Kritpracha B, Pigott JP, Price CI et al (2003) Distal internal iliac artery embolization: a procedure to avoid. J Vasc Surg 37:943–948
Verzini F, Parlani G, Romano L et al (2009) Endovascular treatment of iliac aneurysm: concurrent comparison of side branch endograft versus hypogastric exclusion. J Vasc Surg 49:1154–1161
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chun, JY., Mailli, L., Abbasi, M.A. et al. Embolization of the Internal Iliac Artery Before EVAR: Is It Effective? Is It Safe? Which Technique Should Be Used?. Cardiovasc Intervent Radiol 37, 329–336 (2014). https://doi.org/10.1007/s00270-013-0659-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0659-2