Abstract
Purpose
Our experience with endovascular embolization (EVE) of the bronchial artery (BA) originating from the upper portion of the aortic arch (AA) in six patients is described.
Methods
Altogether, 818 patients with hemoptysis underwent multidetector row computed tomography angiography (MDCTA) before EVE or AA angiography during EVE. Aberrant BAs originating from the upper portion of the AA were the source of massive hemoptysis in six patients (0.73 %). MDCT angiograms and/or Digital subtraction angiograms were retrospectively reviewed. Selective catheterization and embolization were performed.
Results
The ostia of the BAs were located on the superior surface of the AA between the brachiocephalic trunk and left common carotid artery in three patients, the junction of the aorta and medial surface of the left subclavian artery in two, and the posterior wall of the upper portion of the AA in one. The six BAs comprised two common trunks, three single right sides, and one single left side. The targeted vessels were successfully catheterized and embolized by a coaxial microcatheter system using polyvinyl alcohol particles. Other pathologic BAs and nonbronchial systemic arteries also were embolized. Bleeding was immediately controlled in all patients with no recurrence of hemoptysis. No procedure-related complications occurred.
Conclusions
Application of EVE of anomalous origin of BAs in patients with hemoptysis is important, as demonstrated in the six reported patients. MDCTA before EVE or AA angiography during EVE is critical to avoid missing a rare aberrant BA originating from the upper portion of the AA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Massive hemoptysis is a life-threatening condition. Since the report of Remy et al. [1] in 1977, endovascular embolization (EVE) has been well established as an effective means of treating massive hemoptysis. Bronchial, nonbronchial systemic, and pulmonary arteries are possible sources of the bleeding, with the most frequent source the bronchial artery (BA) [1–9]. These vessels should be adequately embolized to ensure effective transcatheter management of hemoptysis [1–11].
The BAs originate directly from the descending thoracic aorta, most commonly at a level between the T5 and T6 vertebrae. Any BA that originates outside this area is considered anomalous, aberrant, or ectopic. The reported prevalence of BAs with an anomalous origin ranges from 8.3 to 36 % [2–4, 12]. Sites of origin of the BAs vary substantially from person to person, which makes identification of the source of bleeding challenging [1–9, 12–16].
BAs originating from the upper portion of the aortic arch (AA)—i.e., the superior 50 % of the arch between the brachiocephalic trunk and the left subclavian artery—are rare [2, 4, 6, 12]. We present six cases in which BAs originating from this region of the AA contributed to massive hemoptysis, which was successfully treated by EVE. The purpose of this study was to evaluate the radiologic findings, imaging response, and embolization of BAs originating from the upper portion of the AA.
Materials and Methods
From May 2009 to September 2012, a total of 818 consecutive patients were referred to our institution for management of hemoptysis using EVE. All patients underwent multidetector computed tomography angiography (MDCTA; n = 259) before EVE or AA angiography (n = 557) during EVE to evaluate the bleeding vessels. We found that BAs originating from the upper portion of the AA were the source of massive hemoptysis in 6 (0.73 %) of the 818 patients.
The patients were all men ranging in age from 23 to 63 (mean 43) years. The maximum volume of hemorrhage was 300–600 (mean 483) mL over 24 h. The underlying etiologies of the hemoptysis in these patients were tuberculosis sequelae (n = 2), bronchiectasis (n = 1), active tuberculosis (n = 1), bronchial–pulmonary arterial fistula (n = 1), and cryptogenic hemoptysis thought to be caused by long-term smoking (n = 1). Two patients had each previously undergone one session of EVE in other institutions, but the bleeding vessel had not been identified.
Our institutional review board approved this study. Patient informed consent was waived for this retrospective study.
The MDCTA was performed with a 64-detector row scanner. A 20-gauge intravenous catheter was inserted into the antecubital vein, and 100 mL of iohexol (350 mg of iodine/mL; Omnipaque, GE Healthcare, Milwaukee, WI) was injected at a rate of 4.0 mL/s. An injection of 30 mL of saline solution followed. An automatic bolus triggering software program was systematically applied, with a circular region of interest positioned at the level of the ascending aorta and a threshold for triggering data acquisition preset at 120 HU.
AA angiography with a 5F (Cook Medical, Bloomington, IN) or 6F (Cordis Corp., Miami Lakes, FL) pigtail catheter with 10 side ports was performed via the femoral artery. This approach included an anteroposterior projection and the pigtail catheter with its tip being located proximal to the origin of the brachiocephalic trunk. An injection of 45 mL of contrast medium (iohexol, 350 mg of iodine/mL) was administered at a rate of 30 mL/s. AA angiography was used to evaluate the BAs and nonbronchial systemic arteries originating from the aorta and bilateral subclavian artery.
Based on the MDCTA or AA angiography findings, all target BAs underwent selective catheterization with a coaxial microcatheter system that used 5F outer catheters (Cook Medical) and 2.6F microcatheters (Asahi Intecc Co. Ltd., Seto, Japan). The location of the ostium and the type of aberrant BA was investigated retrospectively. Embolization with 300–700 μm polyvinyl alcohol particles (Cook Medical) was performed after coaxial microcatheters were advanced into the distal parts of the trunk of the BA. The pathologic BAs and nonbronchial systemic arteries were adequately embolized. Bleeding control, recurrence of hemoptysis, and procedure-related complications in all six patients were investigated retrospectively at the end of February 2013. The mean follow-up period was 10.5 (range 8–27) months.
Results
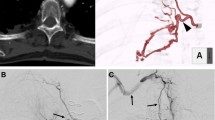
Clinical and radiologic data for the six cases are shown in Table 1. AA angiography showed that the BAs originated from the upper portion of the AA in 4 (0.72 %) of 557 patients, and MDCTA showed BAs of the same origin in 2 (0.77 %) of 259 patients. Thus, aberrant BAs were identified in six patients. AA angiography of four patients revealed two BAs that was definitive (Fig. 1) and two BAs that appeared as an abnormal artery overlapping the upper portion of the AA (Fig. 2). In two cases, MDCTA showed the BAs to be definitive (Fig. 3).
Patient 5. A Angiogram of the aortic arch (AA) shows an enlarged bronchial artery (BA) (arrows). B Selective angiogram with an NIH catheter shows the ostium (star) of the BA on the medial left subclavian artery. C Selective angiogram with a microcatheter (black arrow) shows the BA supplying hypervascular lesions in the right lower lobe (white arrows). D BA after embolization with polyvinyl alcohol particles
Patient 3. A Angiogram of the AA shows an abnormal artery (arrow) overlapping the brachiocephalic trunk. B Tentative angiogram with a Mikaelson catheter shows the site of the ostium (star) on the superior surface of the AA between the brachiocephalic trunk (black arrow) and the left common carotid artery (white arrow). C Selective angiogram with an outer Mikaelson catheter combined with a microcatheter (black arrow) shows a pathologic right BA supplying hypervascular lesions in the right upper lobe (white arrows). D BA after embolization with polyvinyl alcohol particles
Patient 4. A Three-dimensional volume-rendered computed tomography shows the ostium (star) of the BA on the superior surface of the AA between the brachiocephalic trunk and the left common carotid artery. B Selective angiography with an outer Mikaelson catheter combined with a microcatheter shows an enlarged common BA trunk. The pathologic right BA supplies hypervascular lesions in the right upper lobe (arrows)
All of the ostia and the BA types were confirmed by selective catheterization and angiography. The ostia of the BAs were located on the superior surface of the AA between the brachiocephalic trunk and the left common carotid artery in three patients, at the junction of the aorta and medial surface of the left subclavian artery in two patients (Fig. 4), and on the posterior wall of the upper portion of the AA adjacent to the left common carotid artery in one patient (Fig. 5).
Patient 1. A Tentative angiography with a Mikaelson catheter shows a common BA trunk (white arrow) originating from the superior surface of the AA (star) on the medial left subclavian artery (black arrow). B A microcatheter (arrows) advances into the distal part of the BA. C Selective angiography shows the left BA supplying hypervascular lesions in the left upper lobe (arrows). D BA after embolization by polyvinyl alcohol particles
Patient 6. A Three-dimensional volume-rendered computed tomography shows an enlarged right BA (black arrows) originating from the upper part of the AA (star). B–D Transverse maximum-intensity projection computed tomography shows the ostium (star) and course (arrows) of the BA. E Selective angiography with an outer RLG catheter combined with a microcatheter (black arrow) shows a pathologic right BA supplying hypervascular lesions in the right upper lobe (white arrows)
In patients 1 and 4, the BAs comprised two common trunks, with no other BAs. In patients 3, 5, and 6, the BAs comprised the single right sides. Other BAs were composed of: one common trunk and one single left side in patient 3; one right intercostal bronchial trunk and one single left side in patient 5; one common trunk in patient 6. In patient 2, the BA comprised the single left side, with other BAs comprising one right intercostal bronchial trunk and one single left side.
Five of the targeted vessels were successfully catheterized by advancing the curved portion of a Mikaelson (n = 4) or RLG (n = 1) catheter into the AA, including a flexible Streaming microwire (Asahi) and a microcatheter. The final targeted vessel was catheterized by combining an NIH catheter and a coaxial microcatheter system. Six other pathologic BAs, one inferior phrenic artery, and one esophageal artery also were embolized in these six patients. Immediate control of bleeding with no recurrence of hemoptysis was achieved in all patients. Patient 2 had occasional bouts of bloody sputum 15 months after EVE. No procedure-related complications occurred in any of the patients.
Discussion
Cauldwell et al. [12] conducted an extensive anatomic study of BAs in 150 human cadavers. They defined aberrant arteries as those that originate from any area other than the T5-T6 section of the descending aorta. Hartmann et al. [4] performed MDCTA in 214 patients with hemoptysis and reported that 36% of the patients had at least one aberrant BA. The primitive branches originating from the dorsal aortas were initially feeding the pulmonary plexus during embryonic development. Later, the plexus connected to the pulmonary arteries, and the primitive branches began to degenerate. Some of the branches involuted to become adult BAs. The persistence of a primitive bronchial branch of high origin can result in the aberrant BAs that originate from the subclavian, internal mammary, brachiocephalic, vertebral, and carotid arteries, the thyrocervical and costocervical trunks, and the upper portion of the AA [2–4, 6–8, 12–16].
The most common origin of aberrant BAs is the AA, usually at some point between the concavity of the arch and the level of the first aortic intercostal vessels [2–4, 12]. However, BAs originating from the upper portion of the AA are rare. Cauldwell et al. [12] reported only one case of this anatomic variation in 150 human cadavers (0.67 %). We found the variation in 6 (0.73 %) of 818 patients. McPherson et al. [6] reported one case in which an aberrant common BA trunk originated from the superior surface of the AA between the brachiocephalic trunk and the left common carotid artery. In our six cases, three originated from this position, two from the junction of the aorta and the medial surface of the left subclavian artery, and one from the posterior wall of the upper portion of the AA. The six aberrant BAs comprised two common trunks, three single right sides, and one single left side. A single right-sided BA and a right intercostobrachial trunk with this anatomic variation have been reported [2, 4].
Selective catheterization of the BA originating from the superior surface of the AA can be accomplished with a single curved catheter (e.g., Berenstein, DVS, MPA, NIH catheters). In our series, the most successful catheterizations were performed using a Mikaelson catheter. The technique required advancing the curved portion as the outer catheter. This catheter also can be used for catheterization of a BA with a normal origin or an intercostal artery. In the case of the BA originating from the posterior wall of the upper portion of the AA adjacent to the left common carotid artery, successful catheterization was performed using an RLG catheter of a size appropriate to the width of the AA. To avoid the risk of stroke, embolization must be performed carefully, so the microcatheter must enter target vessels that are more distal than usual and the embolic material must be applied slowly.
Thoracic aortography is considered useful to evaluate bleeding vessels in patients with hemoptysis [9, 10]. This method, however, often cannot be used to observe BAs that originate from the upper portion of the AA, brachiocephalic trunk, or the subclavian artery and its branches, among others. MDCTA can more precisely depict the bleeding vessel in the presence of hemoptysis [4, 8, 11]. AA angiography also is more useful than thoracic aortic angiography in emergencies or other situations when patients cannot undergo MDCTA. In our series, four BAs were viewed with AA angiography and two with MDCTA. Immediate control with no recurrence of hemoptysis was achieved in all patients, including two in whom the bleeding vessel had not been identified during the first EVE session.
Conclusions
The variant of BAs originating from the upper portion of AA is rare. Application of EVE in patients of anomalous origin of BAs with hemoptysis is important, as demonstrated here. MDCTA before EVE or AA angiography during EVE is critical to avoid missing this rare aberrant BA.
Abbreviations
- BA:
-
Bronchial artery
- EVE:
-
Endovascular embolization
- AA:
-
Aortic arch
- MDCTA:
-
Multidetector row computed tomography angiography
References
Remy J, Arnaud A, Fardou H, Giraud R, Voisin C (1977) Treatment of hemoptysis by embolization of bronchial arteries. Radiology 122:33–37
Sancho C, Escalante E, Dominguez J, Vidal J, Lopez E, Valldeperas J, Montañá XJ (1998) Embolization of bronchial arteries of anomalous origin. Cardiovasc Intervent Radiol 21:300–304
Yoon W, Kim JK, Kim YH, Chung TW, Kang HK (2002) Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 22:1395–1409
Hartmann IJ, Remy-Jardin M, Menchini L, Teisseire A, Khalil C, Remy J (2007) Ectopic origin of bronchial arteries: assessment with multidetector helical CT angiography. Eur Radiol 17:1943–1953
Yu-Tang Goh P, Lin M, Teo N, En Shen Wong D (2002) Embolization for hemoptysis: a six -year review. Cardiovasc Intervent Radiol 25:17–25
McPherson S, Routh WD, Nath H, Keller FS (1990) Anomalous origin of bronchial arteries: potential pitfall of embolotherapy for hemoptysis. J Vasc Interv Radiol 1:86–88
Uflacker R, Kaemmerer A, Picon PD et al (1985) Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology 157:637–644
Remy-Jardin M, Bouaziz N, Dumont P, Brillet PY, Bruzzi J, Remy J (2004) Bronchial and nonbronchial systemic arteries at multi-detector row CT angiography: comparison with conventional angiography. Radiology 233:741–749
Chun HJ, Byun JY, Yoo SS, Choi BG (2003) Added benefit of thoracic aortography after transarterial embolization in patients with hemoptysis. AJR Am J Roentgenol 180:1577–1581
Phillips S, Ruttley MS (2000) Bronchial artery embolization: the importance of preliminary thoracic aortography. Clin Radiol 55:317–319
Khalil A, Parrot A, Nedelcu C, Fartoukh M, Marsault C, Carette MF (2008) Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT angiography. Chest 133:212–219
Cauldwell EM, Siekert RG, Lininger RE (1948) The bronchial arteries: an anatomic study of 150 human cadavers. Surg Gynecol Obstet 86:395–412
O’Rahilly R, Debson H, Summerfield TK (1950) Subclavian origin of bronchial arteries. Anat Rec 108:227–239
Gailloud P, Albayram S, Heck DV et al (2002) Superior bronchial artery arising from the left common carotid artery: embryology and clinical considerations. J Vasc Interv Radiol 13:851–853
Boyden EA (1970) The developing bronchial arteries in a fetus of the twelfth week. Am J Anat 129:357–368
Amrhein TJ, Kim C, Smith TP et al (2011) Bronchial artery arising from the left vertebral artery: case report and review of the literature. J Clin Imaging Sci 1:62
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, S., Sun, XW., Yu, D. et al. Endovascular Embolization of Bronchial Artery Originating from the Upper Portion of Aortic Arch in Patients with Massive Hemoptysis. Cardiovasc Intervent Radiol 37, 94–100 (2014). https://doi.org/10.1007/s00270-013-0638-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0638-7