Abstract
Purpose
The severity of residual stenosis (RS) sometimes cannot be accurately measured by angiography during central vein intervention. This study evaluated the role of pullback pressure measurement during central vein stenosis (CVS) intervention.
Methods
A retrospective review enrolled 94 consecutive dialysis patients who underwent CVS interventions but not stenting procedures. Patients were classified into 2 groups by either angiography or pressure gradient (PG) criteria, respectively. Groups divided by angiographic result were successful group (RS ≤30 %) and acceptable group (50 % ≥ RS > 30 %), while groups divided by PG were low PG group (PG ≤5 mmHg) and high PG group (PG >5 mmHg). Baseline characteristics and 12-month patency rates between the groups were analyzed.
Results
The angiography results placed 63 patients in the successful group and 31 patients in the acceptable group. The patency rate at 12 month was not statistically different (P = 0.167). When the patients were reclassified by the postintervention pullback PG, the patency rate at 12 months was significant (P = 0.048). Further analysis in groups redivided by different combinations of RS and PG criteria identified significant differences in the group with both RS ≤30 % and PG ≤5 mmHg compared with those with either RS >30 % (P = 0.047) or PG >5 mmHg (P = 0.027). In addition, there was a significant difference between those with both RS ≤30 % and PG ≤5 mmHg compared with those with both RS >30 % and PG >5 mmHg (P = 0.027).
Conclusion
Postintervention PG can better predict long-term outcomes after angioplasty for CVS in nonstented dialysis patients than angiography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central vein stenosis (CVS) is a potentially serious condition with an incidence of 10 to 20 % among hemodialysis patients who undergo interventions for complications related to dialysis access [1, 2]. Recently, percutaneous interventions have been a popular management strategy for CVS as a result of the decreased rate of complications compared with surgical intervention [3–7]. The goals of any intervention are to relieve CVS-related symptoms and maintain dialysis access. According to the guidelines of the National Kidney Foundation—Dialysis Outcomes Quality Initiative (NKF-K/DOQI) [8], the criteria defining success of a technique are <30 % residual stenosis (RS) on angiography and the restoration of dialysis access. In clinical practice, these criteria are also used for CVS interventions. However, two studies suggested that angiography is not predictive of changes in blood flow [9] and may not accurately predict success and patency rates [9, 10]. Whether these criteria are suitable to evaluate the success of CVS interventions remains uncertain.
In coronary artery interventions, angiography criteria defining success are commonly used in clinical practice. However, the limitations of angiography in select cases have led to the development of other tools such as intravascular ultrasound [11] and pressure wire [11–13] to reevaluate the success of a procedure. In particular, the measurement from a pressure wire can influence the coronary intervention strategy [14], and it has been reported to be a better tool for estimating patency rate and complication-free survival than angiography criteria [15]. In cases requiring interventions for dysfunction of dialysis access, intragraft pressure and pressure gradient (PG) at the intragraft stenosis have been used as an alternative to angiography criteria to predict patency rate [16, 17]. However, to our knowledge, no studies have reported the role of pullback PG in CVS interventions. Therefore, this study retrospectively reviewed endovascular CVS interventions in which PG was recorded after the intervention with the aim of evaluating the efficacy and differentiation potency of PG compared with angiography criteria.
Methods
Patient Population
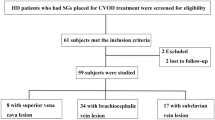
This case-control study surveyed 274 consecutive patients who underwent interventions for CVS from January 2001 to December 2010 in accordance with the recommendations of NKF-K/DOQI [8]. Technical failure was defined as RS >50 % regardless of clinical signs/symptoms. Patients who experienced failed technique, stenting procedures, or percutaneous transluminal angioplasty (PTA) for intrastent lesions were excluded. Postintervention pullback PG was not recorded in every case as the measurement of PG is not essential to the standard technique, and these cases were excluded. Furthermore, patients who did not present at the clinic for follow-up or whose symptoms could not be solved after the intervention were also excluded.
In total, 94 patients with RS ≤50 % on angiography and whose clinical signs/symptoms were solved were selected for further study. These patients were divided into two groups by either angiographic or PG criteria (Fig. 1). Angiography results split the groups into an acceptable group (RS >30 %) and successful (RS ≤30 %) groups [8]. The PG, which was calculated by the receiver operator characteristic curve, established a low PG group (≤5 mmHg) and a high PG group (>5 mmHg). All medical records of the patients who received PTA for CVS were retrospectively reviewed. Details of the interventions were also obtained by reviewing the radiology reports and angiograms. This retrospective study was approved by the internal review board of Chang Gung Memorial Hospital.
Intervention Procedures
Written informed consent was obtained from each patient before the procedure. Diagnostic angiography was performed initially to evaluate the entire dialysis access system and the central vein. Significant stenosis was defined as a reduction in luminal diameter of at least 50 % judged by comparison with the adjacent vessel. When significant stenosis was found, it was crossed by a 0.025- or 0.035-in hydrophilic wire (150 cm length; Terumo, Tokyo, Japan). After a balloon with closed balloon to vessel ratio of 1:1 was positioned across the lesion on the rail of the wire, it was inflated until no waist remained or until the maximal rated balloon pressure was reached, and then dilated for 30–40 s at a time. The size of the balloons used ranged from 8 to 18 mm with a rated burst pressure of 16–30 atm (Wanda, XXL, Boston Scientific Ireland, Galway, Ireland; or Conquest, Bard Peripheral Vascular Inc., Tempe, AZ).
After RS ≤50 % was achieved based on angiography, a 5F Judkins right 4 (JR4) catheter connected to a pressure transducer was pulled back to record the mean pressure across the central vein lesion (Fig. 2A). If the goal, which included RS ≤30 % (technique success), or RS ≤50 % plus PG <10 mmHg, could not be achieved after initial angioplasty, prolonged inflation up to around 2 min was performed. Pullback PG was repeated to evaluate the effect of this prolonged inflation. If RS >50 % persisted, it was considered a technical failure. Two strategies were then pursued: percutaneous transluminal stenting at the CVS was the first choice, and then if the patient refused a stent but had persistent symptoms, surgical closing of the ipsilateral hemodialysis access was carried out.
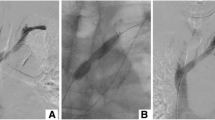
An 80-year-old female dialysis patient accepted an intervention for CVS for right arm swelling. A A 5F JR4 was connected to a pressure transducer. B Angiography demonstrated approximately 90 % stenosis over the brachiocephalic vein. C A 7.0 × 80 mm balloon was used for first dilation. D, G After the first dilation, the RS was approximately 75 %, and the pullback PG was approximately 20 mmHg. E A 14 × 40 mm balloon (approximately 1:1 balloon–vessel ratio) was dilated over the lesion. F, H At the final result, the RS was approximately 50 % but the pullback PG was nearly 0 mmHg. RS residual stenosis, PG pressure gradient
Patient Follow-up
The patients were regularly monitored at our outpatient clinic by nephrologists or a hemodialysis team during the follow-up period. The date of the last follow-up was established as the date of death, the last clinical visit before the end of the study, or the date of any procedure used to treat restenosis. Follow-up data were gathered from clinical records and standardized telephone interviews. Patients were referred back to the Cath Lab for further evaluation if there were any signs or symptoms of restenosis of the central vein or dysfunction of the dialysis access.
Study Definitions
A central vein lesion was defined as stenosis involving the subclavian vein, the brachiocephalic vein, or the superior vena cava. Primary patency rate was defined as the interval between the successful procedure and the first subsequent intervention for restenosis of a central vein lesion [18]. The end point for primary patency assessment was 12 months.
Statistical Analysis
Statistical analysis was performed by SPSS software, version 15 for Windows (IBM, Armonk, NY, USA). Continuous variables were described as mean ± standard deviation, and categorical variables as numbers or percentages. Continuous variables were compared by the two-tailed Student’s t test for parametric data and Mann–Whitney U test for nonparametric data. Categorical variables between 2 groups were compared by the chi-square test. The receiver operator characteristic curve was adopted to calculate a criterion value of the best bound for pullback PG which was defined as the highest sum of sensitivity and specificity (the criterion value on the maximum summation of sensitivity added specificity) in predicting the primary patency rate at 12 months. Correlations between the PG cutoff value and the severity of RS were analyzed by Pearson’s correlation. Primary patency rate was statistically analyzed by the Cox regression curve and life table method. The significance of variables by univariate analysis was determined by binary logistic regression analysis. Follow-up ceased when the patient was lost or died at or before the 1-year follow-up. All P values were two-sided, and statistical significance was set at P ≤ 0.05.
Results
The 94 patients enrolled onto this study were analyzed by comparing groups classified by either angiographic results or postintervention PG.
Groups Divided by Angiography
When all of the patients were divided into two groups by angiographic results, there were 63 cases in the successful group and 31 cases in the acceptable group. There were no statistical differences between the groups in baseline demographic data and the characteristics of dialysis access, with the exception of hypertension (Table 1). Furthermore, there were also no statistical differences in the variables during intervention, except for the degree of RS (24.2 % ± 7.7 vs. 47.8 % ± 8.93; P < 0.001; Table 2). There were no significant differences in 12-month survival (Fig. 3A; P = 0.296; odds ratio [OR] 1.389; 95 % confidence interval [CI] 0.750–2.573) or patency rate at 12 months (Fig. 3A; 54 vs. 39 %; P = 0.167).
Kaplan–Meier plot demonstrating primary patency rates over a 12-month period after endovascular interventions in the different groups. A 12-month primary patency rate of the successful and acceptable groups. B 12-month primary patency rate of PG ≤5 mmHg (Low PG) and PG >5 mmHg (High PG) groups. C 12-month primary patency rate of PG ≤5 mmHg (Low PG) and the successful group. D 12-month primary patency rate of the successful group, PG ≤5 mmHg, and PG 0 mmHg groups. PG pressure gradient
Groups Divided by PG
When all of the patients were divided into two groups by postintervention PG, there were 51 patients in the low PG (PG ≤5 mmHg) group and 43 patients in the high PG (PG >5 mmHg) group. There were no statistical differences between the groups in baseline demographic data, dialysis access, or intervention variables, except for PG (1.7 ± 2.19 vs. 14.3 ± 7.97; P < 0.001; Tables 1 and 2). There was no significant difference in 12-month survival (Fig. 3B; P = 0.126; OR 1.613; 95 % CI 0.875–2.973). However, there was a significant difference in the patency rate at 12 months (Fig. 3B; 60 vs. 37 %; P = 0.048).
Correlation between the Successful Group and Low PG Group
Analysis of the successful group (patients with RS ≤30 %) and low PG group (patients with PG ≤5 mmHg) revealed a correlation of moderate agreement (κ = 0.603). The distributions of baseline characteristics and variables during interventions between the two groups were similar, with the exception of RS (24.2 ± 7.7 % vs. 32.6 ± 15.55 %, P = 0.001) and PG (6.9 ± 7.29 vs. 1.7 ± 2.19, P < 0.001). There was no significant difference in 12-month survival (Fig. 3C; P = 0.698; OR 0.887; 95 % CI 0.484–1.626).
Groups Redivided by Different Combinations of RS and PG Criteria
All of the patients were reanalyzed according to different combinations of RS and PG (Fig. 4). When the patients were grouped by a combination of RS ≤30 % and PG ≤5 mmHg, there were no significant differences in 12-month survival compared with those with RS >30 % (Fig. 4A; P = 0.115; OR 1.812; 95 % CI 0.886–3.790) or PG >5 mmHg (Fig. 4B; P = 0.152; OR 1.771; 95 % CI 0.881–3.870). However, there were significant differences in primary patency rate at 12 months (Fig. 4A, 65 vs. 39 %, P = 0.047; Fig. 4B, 65 vs. 37 %, P = 0.027). In contrast, when the patients were grouped by a combination of RS >30 % and PG >5 mmHg, it revealed a trend in 12-month survival curve compared with those with RS ≤30 % (Fig. 4C; P = 0.089; OR 1.938; 95 % CI 0.903–4.160), or PG ≤5 mmHg (Fig. 4D; P = 0.058; OR 2.173; 95 % CI 0.974–4.844), and also revealed significant differences in primary patency rate at 12 months (Fig. 4C, 54 vs. 23 %; P = 0.050; Fig. 4D, 60 vs. 23 %, P = 0.027).
Kaplan–Meier plot demonstrating primary patency rates over 12-month period in the different groups with different values of RS and PG. A 12-month primary patency rate of cases with RS ≤30 % and PG ≤5 mmHg, compared to those with RS >30 %. B 12-month primary patency rate of cases with RS ≤30 % and PG ≤5 mmHg, compared to those with PG >5 mmHg. C 12-month primary patency rate of cases with RS ≤30 %, compared to those with RS >30 % and PG >5 mmHg. D 12-month primary patency rate of cases with PG ≤5 mmHg, compared to those with RS >30 % and PG >5 mmHg. RS residual stenosis, PG pressure gradient
Discussion
Study Design
In the analysis of dialysis systems, angiography is the standard to define the success of PTA and to decide whether lesions should be treated. According to the DOQI [8], lesions with >50 % stenosis in the dialysis access or central vein can be treated if the hemodialysis patient suffers from access arm swelling or dysfunctional dialysis access. Therefore, this study focused on cases with RS ≤50 % and in whom signs/symptoms were relieved after management. In clinical practice, the severity of a lesion is measured by quantitative coronary arteriography. However, it can be manually adjusted. In contrast, pressure is detected by hemodynamic monitoring systems which cannot be manipulated. In addition, one study suggested that the severity of stenosis detected by angiography cannot well matches that detected by intravascular ultrasound, but it detected by the PG dose [19]. Therefore, this study attempted to determine more scientific parameters to predict the outcome of the procedure, and postintervention PG was selected. If a lesion is fully dilated, the blood flow will not be disturbed and hence the PG should be zero. Initially, we tried to use a PG value of 0 mmHg as a cutoff value; however, the patency rate was nearly the same as the PG ≤5 mmHg group (Fig. 3D). Furthermore, the cutoff value of 5 mmHg provided the highest sensitivity and specificity to differentiate groups in this study, and therefore, 5 mmHg was chosen as the cutoff value. Importantly, anatomic variations in CVS may introduce errors in angiography and affect the estimation of patency. However, stenting is an important factor influencing the patency rate and it would increase the complexity of making comparison of the patency rate in this study. As the group with stenting was small (Fig. 1), we excluded these patients from this study.
Postintervention PG Was Better than RS
The central vein patency rate in the successful group and acceptable group were both comparable to previous studies [5, 7, 18, 20]. Further, the angiographic results were not adequate to differentiate the successful and the acceptable groups (P = 0.296 for the survival curve; P = 0.167 for the patency rate at 12 months). This seems to suggest that an RS of ≤50 % is enough if the clinical signs/symptoms can be resolved by PTA. In contrast, a postintervention PG cutoff value of 5 mmHg represented a better parameter than RS to predict the patency rate and to differentiate the low PG and high PG groups (P = 0.126 for the survival curve; P = 0.048 for the patency rate at 12 months). Of note, our results also indicated that the predictive power of combining both RS and PG criteria was better than a single criterion (either RS or PG), especially when using PG criteria in the acceptable group (Fig. 4D).
Correlation between Postintervention PG and RS
In general, lesions with lower RS values had lower PG. However, the correlation between the successful group and low PG group was just moderate (κ = 0.603) (i.e., Fig. 2 demonstrates a case with RS that did not meet the success criteria; however, the PG was nearly zero after management). Some conditions may explain this result. First, it is sometimes difficult to fill the central vein with contrast medium during angiography as a result of the large size (the reference diameter was around 12 ~ 13 mm in size) which may obscure the vessel margin. Therefore, autodetection of the vessel margin may lead to mistakes in such cases, and the severity of the CVS would need to be manually determined. In addition, stenotic grading is challenging in dialysis access because the reference vessel diameter can be difficult to determine, especially in the central vein [21]. Therefore, the true severity of the CVS is difficult to accurately evaluate in such conditions which may increase the degree of artificial error. Second, the orientation of the central vein can vary (especially in occluded/stenotic central veins), and the shape of the stenotic area is not always circular. This may cause the stenotic lesion to appear either more or less severe than it truly is under fluoroscopy.
Suitability of Devices for Pressure Measurement
In coronary angioplasty, a pressure wire represents an alternative indicator of the success of the procedure [12, 13]. Because the size of the coronary artery is small, the size of pressure wire needs to be small enough to prevent obstructive effects on the PG. In this study, the diagnostic 5F JR4 was selected instead of the pressure wire because of the use of the pressure wire is not usually available in our Cath Lab and the pressure wire is more expensive than the 5F JR4. Further, the outer-sheath diameter of the 5F JR4 is 1.65 mm, and the diameter of a 50 % stenosed central vein lesion is around 6–7 mm according to the measurements in this study. Therefore, the ratio of the area of the central vein to the catheter was approximately 15.5, and the obstructive effect can be ignored. Therefore, the 5F JR4 is a good device to detect pressure on both sides of lesions in the management of CVS.
Limitations
This study has several limitations as a result of its retrospective and investigative characteristics. First, measurement of the PG by catheter pullback across a lesion is not a very accurate method to measure the true difference in pressure across the lesion. A simultaneous 2-catheter technique would have been more accurate. However, the literature suggests that the difference between the two techniques is <2 mmHg [19], and therefore, this difference may have had little effect on our study. On the other hand, venous pressure is easily affected by respiration, and this could increase errors during measurement. The patients were instructed to exhale and then stop respiratory movements during measurement; however, this was difficult to achieve. Second, the small number of cases were insufficient to produce statistically significant results. Third, because no standard surveillance (i.e., regular postintervention sonography) was performed, consistent end points (i.e., the cutoff value of severity or degree of velocity changes of the restenosis area) were hard to achieve. Fourth, pretreated PG and the condition of collaterals were not included in this study. With regard to pretreated PG, we speculate that severe stenosis or even an occluded vessel would increase the ratio of the size of the JR4 catheter to the CVS which would then affect the precise measurement of the PG. Besides, there are currently no studies in the literature on the correlation between PG and clinical symptoms, and it would be difficult to correlate the initial presentations with pretreated PG. With regard to collaterals, they can be found in nearly all patients under initial angiography, and they would be reduced after interventions even if the postintervention PG were high, as illustrated in Fig. 5. Unfortunately, two factors affected the evaluation of collaterals after the interventions in this study. First, the location of the catheter in final angiography was different from that in initial angiography. Second, the final angiography only focused on the central vein lesion in some cases, and so the collaterals were easily missed. Therefore, this variable was excluded and further study may be needed to investigate the correlation between postintervention PG and outcomes. Finally, the postintervention PG measurements were not used for all reinterventions, so comparisons of the assisted patency rates of the different groups could not be performed.
Collaterals during intervention for central vein stenosis. A, B Collaterals in a patient with a postintervention PG of 0. A Many collaterals under the subclavian vein was occluded. B Reduced collaterals were noted after intervention. C,D Collaterals in a patient with a postintervention PG of approximately 20 mmHg. C Many collaterals under the subclavian vein were occluded. D The collaterals were also diminished after intervention. PG pressure gradient
Conclusions
The results of this study indicated that the 12-month patency rates in both the successful and acceptable groups were compatible with previous studies. Therefore, a severity of RS ≤50 % can be considered acceptable after relief of the clinical signs/symptoms. A postintervention PG of 5 mmHg had a better differentiating potency than the value of 30 % RS during angioplasty for CVS. Therefore, we suggest that postintervention pullback PG is a better alternative to predict the long-term outcomes after interventions for CVS in nonstented dialysis patients, and that it is a good tool even in cases that are not in accordance with the conventional standard criteria.
References
Nael K, Kee ST, Solomon H, Katz SG (2009) Endovascular management of central thoracic veno-occlusive diseases in hemodialysis patients: a single institutional experience in 69 consecutive patients. J Vasc Interv Radiol 20:46–51
Bozof R, Kats M, Barker J, Allon M (2007) Time to symptomatic vascular stenosis at different locations in patients with arteriovenous grafts. Semin Dial 21:285–288
Maya ID, Saddekni S, Allon M (2007) Treatment of refractory central vein stenosis in hemodialysis patients with stents. Semin Dial 20:78–82
Oderich GS, Treiman GS, Schneider P, Bhirangi K (2000) Stent placement for treatment of central and peripheral venous obstruction: a long-term multi-institutional experience. J Vasc Surg 32:760–769
Kim YC, Won JY, Choi SY et al (2009) Percutaneous treatment of central venous stenosis in hemodialysis patients: long-term outcomes. Cardiovasc Intervent Radiol 32:271–278
Masková J, Komárková J, Kivánek J et al (2003) Endovascular treatment of central vein stenoses and/or occlusions in hemodialysis patients. Cardiovasc Intervent Radiol 26:27–30
Aytekin C, Boyvat F, Yagmurdur MC et al (2004) Endovascular stent placement in the treatment of upper extremity central venous obstruction in hemodialysis patients. Eur J Radiol 49:81–85
Vascular Access Work Group (2006) Clinical practice guidelines for vascular access. Am J Kidney Dis 48:S248–S273
Ahya SN, Windus DW, Vesely TM (2001) Flow in hemodialysis grafts after angioplasty: do radiologic criteria predict success? Kidney Int 59:1974–1978
Clark TW, Hirsch DA, Jindal KJ et al (2002) Outcome and prognostic factors of restenosis after percutaneous treatment of native hemodialysis fistulas. J Vasc Interv Radiol 13:51–59
Hanekamp CE, Koolen JJ, Pijls NH et al (1999) Comparison of quantitative coronary angiography, intravascular ultrasound, and coronary pressure measurement to assess optimum stent deployment. Circulation 99:1015–1021
Fischer JJ, Samady H, McPherson JA et al (2002) Comparison between visual assessment and quantitative angiography versus fractional flow reserve for native coronary narrowings of moderate severity. Am J Cardiol 90:210–215
Hirota M, Iwasaki K, Yamamoto K et al (2006) Coronary pressure measurement to identify the lesion requiring percutaneous coronary intervention in equivocal tandem lesions. Coron Artery Dis 17:181–186
Sant’Anna FM, Silva EE, Batista LA et al (2007) Influence of routine assessment of fractional flow reserve on decision making during coronary interventions. Am J Cardiol 99:504–508
Wongpraparut N, Yalamanchili V, Pasnoori V et al (2005) Thirty-month outcome after fractional flow reserve-guided versus conventional multivessel percutaneous coronary intervention. Am J Cardiol 96:877–884
Lai CC, Chung HM, Tsai HL et al (2010) Intragraft pressures predict outcomes in hemodialysis patients with graft outflow lesions undergoing percutaneous transluminal angioplasty. Catheter Cardiovasc Interv 76:206–211
Funaki B, Kim R, Lorenz J et al (2002) Using pullback pressure measurements to identify venous stenoses persisting after successful angioplasty in failing hemodialysis grafts. Am J Roentgenol 178:1161–1165
Renaud CJ, Francois M, Nony A et al (2012) Comparative outcomes of treated symptomatic versus non-treated asymptomatic high-grade central vein stenoses in the outflow of predominantly dialysis fistulas. Nephrol Dial Transplant 27:1631–1638
Labropoulos N, Borge M, Pierce K, Pappas PJ (2007) Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg 46:101–107
Quinn SF, Schuman ES, Demlow TA et al (1995) Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol 6:851–855
Sampson JJ, Saunders JB, Capp CS (1940) Compression of the subclavian vein by first rib and clavicle. Am Heart J 19:292–315
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, YS., Yang, CH., Chu, CM. et al. The Role of Postintervention Pullback Pressure Gradient in Percutaneous Transluminal Angioplasty for Central Vein Stenosis in Dialysis Patients. Cardiovasc Intervent Radiol 36, 1296–1305 (2013). https://doi.org/10.1007/s00270-013-0548-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-013-0548-8