Abstract
Purpose
Although the majority of iatrogenic pseudoaneurysms (PSAs) are amenable to ultrasound (US)-guided thrombin injection, patients with those causing neuropathy, claudication, significant venous compression, or soft tissue necrosis are considered poor candidates for this option and referred to surgery. We aimed to test the effectiveness and feasibility of a novel percutaneous cyanoacrylate glue (NBCA-MS)-based technique for treatment of symptomatic and asymptomatic iatrogenic PSA.
Material and Methods
During a 3-year period, we prospectively enrolled 91 patients with iatrogenic PSA [total n = 94 (femoral n = 76; brachial n = 11; radial n = 6; axillary n = 1)]. PSA were asymptomatic in 66 % of cases, and 34 % presented with symptoms due to neuropathy, venous compression, and/or soft tissue necrosis. All patients signed informed consent. All patients received NBCA-MS-based percutaneous treatment. PSA chamber emptying was first obtained by US-guided compression; superior and inferior walls of the PSA chamber were then stuck together using NBCA-MS microinjections. Successfulness of the procedure was assessed immediately and at 1-day and 1-, 3-, and 12-month US follow-up.
Results
PSA occlusion rate was 99 % (93 of 94 cases). After treatment, mean PSA antero-posterior diameter decrease was 67 ± 22 %. Neuropathy and vein compression immediately disappeared in 91 % (29 of 32) of cases. Patients with tissue necrosis (n = 6) underwent subsequent outpatient necrosectomy. No distal embolization occurred, nor was conversion to surgery necessary.
Conclusion
PSA treatment by way of NBCA-MS glue injection proved to be safe and effective in asymptomatic patients as well as those with neuropathy, venous compression, or soft-tissue necrosis (currently candidates for surgery). Larger series are needed to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iatrogenic pseudoaneurysms (PSAs) have been reported to complicate 0.1–1 % of diagnostic angiography and up to 2.6 % of interventional procedures [1–3]. Percutaneous thrombin injection is currently the first-line treatment for arterial catheterization-site PSA. However, patients presenting with mass effect (nerve, vein compression, and soft-tissue necrosis) are currently referred for surgical treatment because thrombin injection does not yield immediate decrease of PSA volume and converts it into a nonpulsating mass [4–10]. Furthermore, spillage of thrombin in the native artery is not uncommon [11], and the low incidence of clinically significant embolic or thrombotic complications may be explained by spontaneous endogenous fibrinolysis [6, 12–15].
In 2003, Aytekin et al. [16] first described the use of a cyanoacrylate glue to induce PSA thrombosis as an alternative to thrombin injection. However, this procedure, which has not been further validated, turned the PSA chamber into a thrombotic nonpulsating mass as observed with thrombin injection.
This raises the need for novel techniques of percutaneous treatment of iatrogenic PSA to elicit decrease of the PSA chamber volume and possibly limit the rate of surgical referral for symptomatic patients. A decrease in PSA volume can be obtained through the ejection of blood from the PSA chamber by means of ultrasound (US)-guided PSA compression. Nonetheless, persistent adhesion of the collapsed PSA chamber walls is reasonably expected with only the use of a glue acting on solid tissues, rather than with thrombin injection, the effect of which is to induce blood thrombosis.
We thus aimed to test the effectiveness and feasibility of percutaneous treatment of iatrogenic PSA based on microinjections of cyanoacrylate glue [n-butyl-2-cyanoacrylate + comonomer (NBCA-MS)] after US-guided PSA compression.
Materials and Methods
Study Population
Between February 2008 and February 2011, 104 consecutive patients referred to our department for iatrogenic PSA management were prospectively screened for percutaneous NBCA-MS-based treatment.
Inclusion criteria were the presence of post-arterial catheterization iatrogenic PSA with maximum diameter (including thrombus and chamber) >3 cm. PSA with maximum diameter ≤3 cm were also included if they were symptomatic or persisted 2 months after completion of the indicated dual antiplatelet therapy period. The impossibility to collapse PSA by US probe and to exclude flow from the PSA chamber, as well as evidence of a ruptured or infected PSA, were the only exclusion criteria.
The study was performed according to the Declaration of Helsinki and approved by the Institutional Ethics Committee. All patients were aware of the therapeutic options and signed an informed consent.
PSA Biometry
Preprocedural US evaluation included assessment of the following parameters: arterial defect diameter; neck length; PSA longitudinal, antero-posterior, and latero-lateral diameters; PSA chamber diameter; and presence of arteriovenous fistula. PSA were arbitrarily defined as “large” when the maximum diameter was ≥5 cm. The PSA chamber was arbitrarily defined as “large” when the maximum diameter was ≥4 cm.
Cyanoacrylate Glue
NBCA-MS (Glubran2; General Enterprise Marketing, Viareggio, Italy) is an n-butyl cyanoacrylate glue comprising a proprietary comonomer as approved by the European Community for internal human use.
This synthetically derived glue shows rapid consolidation/polymerization (1–5 s), but complete sealing occurs in approximately 5 min. It can be used in its pure form, with no need for contrast medium, which has shown to prolong consolidation time. Moreover, NBCA-MS presents high echogenicity and for this reason it is easily detectable by US when injected in vessels or tissues. Finally, given its high effectiveness in eliciting thrombus/tissue consolidation (up to twice the volume of the injected glue), only small amounts of glue are required. The glue also exhibits antiseptic properties [17], has efficacy that is not affected by antiplatelet or anticoagulant therapy, and has lower temperatures of polymerization compared with other NBCAs [18].
Procedure
All patients received a preliminary injection of 1 ml 2 % xylocaine near the femoral, brachial, or radial nerves under US control. Likewise, 0.5 ml 2 % xylocaine was injected just superficial to the PSA.
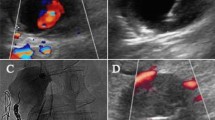
We first induced the collapse of the PSA chamber by means of a US probe. Once complete collapse was obtained, no blood was detectable inside the PSA chamber, and the superior and inferior PSA walls were superimposed, constituting a single hypoechogenic layer plugging the ostium of the PSA chamber (Figs. 1, 2). Appropriate PSA chamber collapse was confirmed by the complete disappearance of flow inside the PSA chamber. Under strict US control, a 22-G needle was thus positioned within the hypoechogenic area and 0.2 ml NBCA-MS (two boluses of 0.1 ml each using two different needles and syringes) were injected (Fig. 3).
Cyanoacrylate glue injection. Schematic representation (left panel) and US imaging (right panel) of the procedure of glue injection inside the compressed PSA. A brachial artery, T hypoechogenic tissue constituted by the superimposed superior and inferior PSA chamber walls, N tip of the needle, G glue injected inside the hypoechogenic area, P US probe compressing the PSA
To prevent needle occlusion, the glue was first drawn into a 2.5-ml syringe, the needle was then removed, and a new needle positioned and filled with 5 % w/v glucose solution. After glue injection, compression was maintained for 5 min. Additional glue injection (0.1 ml in ≤3 injections) was performed during the same procedure if signs of PSA reperfusion were observed after slight release from probe compression. Finally, a pressure dressing was applied, and 10 min of patient bed rest were required.
In case of large PSA with a large chamber, slow compression was performed after preliminary US-guided introduction of a 14G needle into the PSA cavity to obtain complete emptying of the PSA chamber. At discharge, patients were recommended to avoid strenuous activity for 24 h.
The success of the procedure, defined as no observed sign of perfused PSA detectable by US, was assessed immediately after glue injection and at 1 day and again at 1, 3, and 12 months after the procedure (Fig. 4). Procedure time was measured from the induction of local anesthesia until the last glue injection.
Statistical Analysis
Statistical analysis was performed by SPSS 13.0 software (SPSS, Chicago, IL). Values are presented as means ± SDs for variables with normal distribution. For continuous variables, comparison between groups were made by one-way analysis of variance with Bonferroni post hoc correction when appropriate. Categorical variables were compared using Fisher’s exact test, and p < 0.05 was considered statistically significant.
Results
Patients Characteristics
Of the overall screened population, 13 patients presented with ruptured PSA and were excluded from the study. We thus treated 91 patients (50 men, 41 women; age 70 ± 11 years) affected by iatrogenic PSA with NBCA-MS injections [total n = 94 (femoral n = 76; brachial n = 11; radial n = 6; axillary n = 1)]. The features of the treated PSAs are listed in Table 1.
The mean maximum PSA diameter was 49 ± 20 mm (range 22–120), and 10 PSAs had maximum diameter <3 cm. Neck length ranged from 0 to 3 cm; arterial defect width ranged from 1.5 to 5.0 mm. We observed 61 simple PSA with one lobe and 33 polylobate PSAs. Three patients presented two PSAs with two distinct necks originating from the same artery. In ten cases (10.6 %), the PSAs were large and presented with a large chamber and thus underwent preliminary emptying by means of needle aspiration. In three cases, an arterial-venous fistula was associated with PSA. Because fistulas were not high-output and patients were on dual antiplatelet therapy, we decided to defer the surgical correction of fistulas and to perform percutaneous treatment of PSAs that were instead symptomatic.
Of 32 patients (34 %), 25 presented with neuropathy only, six presented with neuropathy plus soft-tissue necrosis, and one presented with neuropathy plus vein compression. Interestingly, patient age, sex, and background antiplatelet/anticoagulant therapy did not influence PSA size, whereas femoral arterial catheterization and wider arterial defect diameters were associated with larger PSA. In 17 cases (18 %), patients required immediate additional glue injection. The mean amount of injected glue was 0.23 ± 0.12 ml. Needle occlusion occurred in 11 cases (12 %) and required needle substitution.
Outcomes of the Procedure
PSA diameter decrease immediately after the procedure was 67 ± 22 % in the antero-posterior diameter, which, unlike latero-lateral and longitudinal diameter, is influenced by PSA compression. Mean time of treatment was 14 min (range 7–30).
The immediate, 1-day and 1-month success rates of the procedure were 100 % (94 of 94). The late success rate of the procedure was 99 % (93 of 94) because residual PSA was detectable at 3 months by US scan in only one asymptomatic patient who underwent retreatment and showed no detectable PSA at 12-month US follow-up.
Patients with PSA complicated by mass effect experienced immediate relief from symptoms in 91 % (29 of 32) of cases. One patient complained of persistent, mild symptoms after the procedure, which were self-limiting within 7 days. Owing to the presence of brachial nerve injury (as shown by electromyography), pain persisted in one patient. Subjects with associated tissue necrosis (n = 6) underwent outpatient necrosectomy 3 days after PSA percutaneous treatment; healing of the skin lesions occurred within 2 months.
Safety of the Procedure
Patients required neither sedation nor anaesthesiology support. No distal embolization of thrombotic material was observed during PSA compression. No bleeding, glue embolization, native artery stenosis or thrombosis, allergic reaction, vein thrombosis, infection, or PSA rupture were observed, nor was conversion to surgery needed.
Discussion
US-guided thrombin injection has become the treatment of choice for iatrogenic PSA [4, 5, 7, 9, 11, 19, 20]. However, patients with PSA complicated by infection, rapid expansion, neuropathy, vein compression, or soft-tissue necrosis, the incidence of which is unclear [9], are still usually referred to surgical management [4–7, 10]. Furthermore, it is not yet clear whether PSA biometry, neck length, chamber, and arterial defect diameter might influence effectiveness and rates of complication, such as thrombin leakage into the artery [4–6, 12, 21]. We have thus tested the safety and effectiveness of NBCA-MS-based PSA treatment in both symptomatic and asymptomatic patients.
Effectiveness of the Procedure in Asymptomatic and Symptomatic Patients
In the present series NBCA-MS injection proved to be effective independently of PSA biometry (even in PSAs with no neck, wide arterial defect, or PSA chamber). Interestingly, NBCA-MS injection has been successful even in PSA complicated by mass effect (currently referred to surgical decompression) [22] because, unlike thrombin injection, it allows the surgeon to obtain the disappearance of the PSA chamber instead of its thrombosis. This might also explain the effectiveness of the NBCA-MS-based technique in inducing relief from symptoms in patients presenting with PSA having mass effect.
Failure of PSA treatment was observed in one patient (1 vs. 2 % reported for thrombin injection) [7, 8, 12, 23], who underwent coronary stenting and aggressive antiplatelet therapy 3 days after the procedure. Likewise, the complication rate and need for multiple glue reinjections during the same procedure were as frequent as seen with thrombin injection (0 vs. 0–4 % and 18 vs. 4–37 %, respectively) [4, 7, 13, 14, 24, 25].
Technical Peculiarity of Glue-Based PSA Treatment
To our knowledge, Aytekin et al. [16] first described the use of cyanoacrylate glue for treatment of PSA. However, they employed the glue as an alternative to thrombin to induce thrombosis of the PSA chamber [26]. In contrast, we induced collapse of the PSA chamber and then stuck its superior and inferior walls together. We thus obtained the disappearance of the PSA chamber with this being the main novelty of the present technique.
Due to its capillary action, NCBA-MS consolidates a volume of thrombus/tissue up to twice its own volume. Therefore, small amounts of glue (0.1 ml/injection [mean amount of glue injected 0.24 vs. 0.5 ml/injection; mean 1.5 ml used by Aytekin) diffused within the hypoechogenic layer and were sufficient to maintain adhesion of the superior to the inferior wall of the PSA. The exclusion of blood flow across the ostium of the PSA chamber, as well as the injection of glue within solid tissues (where glue can slowly diffuse) rather than in the PSA chamber, is likely to decrease potential distal embolization.
Finally, unlike the technique described by Aytekin, we did not mix cyanoacrylate glue, which presents high echogenicity, with contrast medium (e.g., Lipiodol) because it prolongs consolidation time.
Study Limitations
Some limitations of the current study must be addressed. First, data on sheath diameter were available for only a minor portion of endovascular procedures. Furthermore, arterial defect was rarely detectable by US only; arterial defect diameter was thus assessed by means of color Doppler, which is likely to overestimate the measurements. Finally, a limitation of the present study is represented by the lack of a control arm. In particular, we could envisage a future study comparing the safety and efficacy of cyanoacrylate glue and thrombin injection in the treatment of patients with iatrogenic PSA.
Conclusion
Our study shows that given the presence of a collapsible PSA, NBCA-MS-based treatment is a feasible approach independent of PSA diameter, presence/absence of neck, and arterial defect diameter. It is worth noting that this percutaneous technique, inducing disappearance of the PSA chamber, has been successful in PSA complicated by nerve/vein compression and also tissue necrosis, for both of which surgery currently represents the treatment of choice, and may extend percutaneous treatment to a subset of symptomatic patients. This may hold significant socioeconomic implications in the current management of patients with iatrogenic PSA. Future studies based on larger populations are thus needed to confirm the safety and effectiveness of this procedure and to compare it with current indicated percutaneous and surgical approaches.
References
Biancari F, D’Andrea V, Di Marco C et al (2010) Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J 159(4):518–531
Demirbas O, Batyraliev T, Eksi Z et al (2005) Femoral pseudoaneurysm due to diagnostic or interventional angiographic procedures. Angiology 56(5):553–556
Bangalore S, Bhatt DL (2011) Femoral arterial access and closure. Circulation 124(5):e147–e156
Hanson JM, Atri M, Power N (2008) Ultrasound-guided thrombin injection of iatrogenic groin pseudoaneurysm: doppler features and technical tips. Br J Radiol 81(962):154–163
Morgan R, Belli AM (2003) Current treatment methods for post catheterization pseudoaneurysms. J Vasc Interv Radiol 14(6):697–710
Ferguson JD, Whatling PJ, Martin V et al (2001) Ultrasound guided percutaneous thrombin injection of iatrogenic femoral artery pseudoaneurysms after coronary angiography and intervention. Heart 85(4):4–5
Krueger K, Zaehringer M, Strohe D et al (2005) Post catheterization pseudoaneurysm: results of US-guided percutaneous thrombin injection in 240 patients. Radiology 236(3):1104–1110
Hung B, Gallet B, Hodges TC (2002) Ipsilateral femoral vein compression: a contraindication to thrombin injection of femoral pseudoaneurysms. J Vasc Surg 35(6):1280–1283
Webber GW, Jang J, Gustavson S et al (2007) Contemporary management of postcatheterization pseudoaneurysms. Circulation 115(20):2666–2674
Edgerton JR, Moore DO, Nichols D et al (2002) Obliteration of femoral artery pseudoaneurysm by thrombin injection. Ann Thorac Surg 74(4):1413–1415
Grewe PH, Mügge A, Germig A et al (2004) Occlusion of pseudoaneurysms using human or bovine thrombin contrast-enhanced ultrasound guidance. Am J Cardiol 93(12):1540–1542
Paulson EK, Nelson RC, Mayer CE et al (2001) Sonographically guided thrombin injection of iatrogenic femoral pseudoanerurysms: further experience of a single institution. Am J Roentgenol 177(2):309–316
Sackett WR, Taylor SM, Coffey CB et al (2000) Ultrasound-guided thrombin injection of iatrogenic femoral pseudoaneurysms: a prospective analysis. Am Surg 66(10):937–940
Kang SS, Labropoulos N, Mansour MA et al (1998) Percutaneous ultrasound guided thrombin injection: a new method for treating post catheterization femoral pseudoaneurysms. J Vasc Surg 27(6):1032–1038
Oholow MA, Secknus MA, Von Korn H et al (2008) Percutaneous thrombin injection for treatment of iatrogenic femoral artery pseudoaneurysms: a case for caution. Angiology 59(3):372–375
Aytekin C, Firat A, Gültekin B et al (2003) A US-guided glue injection in the treatment of femoral pseudoaneurysms. Tani Girisim Radyol 9(2):257–259
Montanaro L, Arciola CR, Cenni E et al (2001) Cytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical use. Biomaterials 22(1):59–66
Heye S, Maleux G, Wilms G (2006) Pain experience during internal spermatic vein embolization for varicocele: comparison of two cyanoacrylate glues. Eur Radiol 16(1):132–136
Righini M (2007) Post-catetherization false femoral aneurysms. Rev Med Suisse 3(97):341–345
Hajarizadeh H, La Rosa CR, Cardullo P et al (1995) Ultrasound-guided compression of post-catheterization femoral pseudoaneurysm failure, recurrence, and long-term results. J Vasc Surg 22(4):425–433
La Perna L, Olin JW, Goined D et al (2000) Ultrasound-guided thrombin injection for the treatment of post catheterization pseudoaneurysms. Circulation 102(19):2391–2395
Saad NE, Saad WE, Davies MG et al (2005) Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics 25:173–189
Stone PA, Abu Rahma AF, Flaherty SK et al (2006) Femoral pseudoaneurysms. Vasc Endovasc Surg 40(2):109–117
Mohler ER III, Mitchell ME, Carpenter JP et al (2001) Therapeutic thrombin injection of pseudoaneurysms: a multicenter experience. Vasc Med 6(4):241–244
Paulson EK, Sheafor DH, Kliewer MA et al (2000) Treatment of iatrogenic femoral arterial pseudoaneurysms: comparison of US-guided thrombin injection with compression repair. Radiology 215(2):403–408
Aytekin C, Firat A, Yildirim E et al (2004) Ultrasound-guided glue injection as alternative treatment of femoral pseudoaneurysms. Cardiovasc Intervent Radiol 27(6):612–615
Acknowledgments
The authors thank Michele Emdin for critical revision of the manuscript.
Conflict of interest
Drs. Andrea Del Corso and Giuseppe Vergaro have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Corso, A., Vergaro, G. Percutaneous Treatment of Iatrogenic Pseudoaneurysms by Cyanoacrylate-Based Wall-Gluing. Cardiovasc Intervent Radiol 36, 669–675 (2013). https://doi.org/10.1007/s00270-012-0502-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-012-0502-1