Abstract
Purpose
Avoidance of nontarget microsphere deposition via hepatoenteric anastomoses is essential to the safety of yttrium-90 radioembolization (RE). The hepatic hilar arterial network may remain partially patent after coil embolization of major arteries, resulting in persistent risk. We retrospectively reviewed cases where n-butyl cyanoacrylate (n-BCA) glue embolization was used to facilitate endovascular hepatic arterial skeletonization before RE.
Methods
A total of 543 RE procedures performed between June 2004 and March 2012 were reviewed, and 10 were identified where n-BCA was used to embolize hepatoenteric anastomoses. Arterial anatomy, prior coil embolization, and technical details were recorded. Outcomes were reviewed to identify subsequent complications of n-BCA embolization or nontarget RE.
Results
The rate of complete technical success was 80 % and partial success 20 %, with one nontarget embolization complication resulting in a minor change in treatment plan. No evidence of gastrointestinal or biliary ischemia or infarction was identified, and no microsphere-related gastroduodenal ulcerations or other evidence of nontarget RE were seen. Median volume of n-BCA used was <0.1 ml.
Conclusion
n-BCA glue embolization is useful to eliminate hepatoenteric networks that may result in nontarget RE, especially in those that persist after coil embolization of major vessels such as the gastroduodenal and right gastric arteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complications resulting from hepatic radioembolization (RE) are widely reported with a significant proportion as a result of nontarget deposition of yttrium-90 (Y-90) microspheres in the gastrointestinal tract, skin, and lungs [1, 2]. Such complications, including gastrointestinal ulcers and radiation dermatitis, limited the early adoption of RE starting in the 1960s [3, 4]. Since then, dramatic improvements in angiographic imaging quality and in catheter technology have allowed depiction and management of culpable hepatoenteric anastomoses.
The convention most widely accepted is to perform the endovascular equivalent of surgical skeletonization of the hepatic arteries via embolization of hepatoenteric anastomoses [5, 6]. Skeletonization almost never causes ischemia, because macroscopic embolization of major vessels induces immediate formation of collateral flow to the affected vascular beds. When the collateral flow originates from the superior mesenteric or other nonhepatic circulation, skeletonization is achieved. However, collateral flow may also be recruited from other hepatofugal vessels supplying the hilar peribiliary network [7]. These small vessels may be extremely challenging to catheterize and to embolize, and appear to increase the risk of ulceration. We report our experience of using n-butyl cyanoacrylate (n-BCA) glue to embolize these hepatoenteric anastomotic networks.
Materials and Methods
All data were handled in accordance with the Health Insurance Portability and Accountability Act. The institutional review board approved this retrospective study and granted a waiver from obtaining patient consent.
All RE procedures performed before March 2012 at a single center were retrospectively reviewed. All cases were identified where vessels were catheterized and embolized with n-BCA to facilitate skeletonization of the hepatic arteries, either during preparatory or treatment sessions.
In each n-BCA embolization patient, microcatheter selection was performed of branch arteries suspected of supplying extra-hepatic tissues. Digital subtraction angiography was performed with careful attention paid to the volume and rate of injection required before reflux occurred. Following flushing of the catheter with 5 % dextrose solution, a volume of approximately 0.2 ml of 3:1 lipiodol:n-BCA glue mixture was loaded into the microcatheter lumen. Under magnification digitally subtracted fluoroscopy (road map), the lipiodol/glue mixture was extruded from the catheter using dextrose solution in a 1-ml syringe. Injection was halted when the lipiodol:n-BCA mixture filled the target bed back to the catheter tip.
Upon retrospective review, the number and locations of hepatoenteric vessels, agents used for embolization, and appearance or reappearance of new anastomoses were recorded. The size, type, and position of microcatheters used, the lipiodol:n-BCA ratio, and volume used for embolization were noted for each subject. The locations of RE microsphere administration proximal or distal to the sites of n-BCA embolization were also recorded.
Thorough chart review was performed on each patient to identify any subsequent complications of n-BCA embolization or of nontarget RE, including review of 1- and 3-month and subsequent clinic notes, 2-, 4-, 8-, and 12-week and later laboratory results, and 2- to 3-month and all subsequent follow-up imaging. If endoscopy and/or biopsy were performed, the histopathology reports were reviewed in each case with attention to the presence of ischemic changes and/or microspheres.
Results
A total of 543 procedures performed on 267 patients between June 2004 and March 2012 were reviewed. Of these, 10 procedures on 10 patients were identified where n-BCA was used to achieve skeletonization of the hepatic artery (Table 1). All patients were treated for multifocal, bilobar, metastatic malignancies, and underwent single session whole-liver or whole-liver remnant treatment with resin microspheres (SIR-Spheres, Sirtex, Inc., Lane Cove, Australia), with a single administration into the proper hepatic artery (PHA) or common hepatic artery (CHA) (n = 5) or with separate lobar administrations (n = 5).
All of the arteries embolized with n-BCA were small, measuring <1 mm in diameter, requiring the use of a 1.9–2.4 French microcatheter for selection. Of 10 n-BCA embolized vessels, 9 supplied the perihilar biliary ductal plexus, including 8 supraduodenal arteries supplying the superior duodenum [8] (Fig. 1), and 1 right gastric artery (RGA) arising from the PHA giving off proximal branches to the biliary plexus (Fig. 2). In addition, 1 accessory left inferior phrenic artery arising from the left hepatic artery (LHA) in a plexiform pattern was embolized with use of both a single coil and n-BCA.
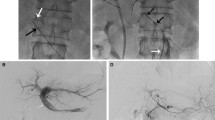
A 46-year-old man with metastatic colon cancer underwent preparatory angiography and skeletonization with coil embolization of the gastroduodenal artery (GDA) and right gastric artery (RGA). A Treatment angiography 15 days later demonstrated interval appearance of a supraduodenal artery (SDA, arrow) arising from the proper hepatic artery (PHA). B Selective angiography using a 2.3F J-tip microcatheter confirmed parenchymal enhancement of the duodenum, but the catheter could not be further advanced. C The glue cast after n-butyl cyanoacrylate (n-BCA) embolization filled a large portion of the network. D Repeat angiography revealed successful skeletonization with no additional hepatoenteric communications. This patient was treated with 2.09 GBq resin microspheres via the PHA without complications
A 62-year-old man with metastatic leiomyosarcoma became refractory to systemic therapies. A Preparatory hepatic angiogram revealed a small right gastric artery (RGA) with a sharply angulated origin (arrow). Retrograde access to the RGA via catheterization of the left gastric artery was not technically feasible because of an incomplete arcade. B A 2.3F J-tip microcatheter was formed into a Waltman loop to select the small RGA but could not be advanced further. C Postembolization angiography revealed n-butyl cyanoacrylate (n-BCA) glue cast and no residual visible hepatoenteric anastomoses. D Angiogram at the time of treatment 7 days later revealed interval appearance of a new supraduodenal artery (SDA; arrow) adjacent to the glue cast, which could not be catheterized. Thus, the n-BCA embolization was considered only a partial success, and this patient received a 2.30 GBq resin microsphere dose delivered in the proper hepatic artery (PHA) distal to the SDA using an antireflux microcatheter (Surefire Infusion System, SureFire Medical, Inc., Westminster, CO) without complications
Sharp angulation and small caliber of target vessels limited microcatheter purchase in all cases, with the median depth of catheter insertion of 4.6 mm (range 1.5–24 mm) (Fig. 2). Instability of microcatheter positioning precluded safe coil embolization in almost all cases. In addition, n-BCA was chosen in these cases to treat complex networks that would not be entirely eliminated by use of coils alone (Figs. 1, 3).
Arterial anatomy of the hepatic hilum and extrahepatic biliary tree. An innumerable variety of configurations may exist to supply arterial blood to this region. The anatomy consists of a network with many possible inflows. Because the arteries do not have valves, flow is reversible in each of them. Disruption of flow in any branch, such as by coil embolization, surgical ligation, or tumor invasion, promotes collateral inflow from other sources. Smaller crosslinks are intentionally omitted in this diagram for clarity. The supraduodenal artery, sometimes known as the 3 o’clock and 9 o’clock arteries, or erroneously as the retroportal artery, may originate from the cystic, right hepatic, proper hepatic, left hepatic, or right gastric arteries, as shown. The posterior superior pancreaticoduodenal artery is also known as the retroduodenal artery [8, 13, 16–22]. CH common hepatic, PH proper hepatic, LH left hepatic, RH right hepatic, ILC interlobar communicating, DP dorsal pancreatic, GD gastroduodenal, ASPD anterior superior pancreaticoduodenal, PSPD posterior superior pancreaticoduodenal, RD retroduodenal, RGE right gastroepiploic, RD retroduodenal, RG right gastric, C cystic, DC deep cystic, SC superficial cystic, SD supraduodenal
The branches embolized for skeletonization of the hepatic artery (elimination of hepatofugal hepatoenteric anastomoses) are listed in Table 2. Target vessels for n-BCA embolization were identified and embolized during pretreatment preparatory angiography in 7 patients and during treatment administration angiography in 3. Technical details of n-BCA embolizations are listed in Table 3. The majority of cases required not only small caliber microcatheters but also preshaped catheters. A 2.3F J-tip Prowler Plus microcatheter (Codman Neurovascular/DuPuy, Raynham, MA) was used in 6 of 10 cases. Complete angiographic skeletonization was accomplished in 8 patients, and partial success was accomplished in 2 patients, in whom residual or new hepatoenteric anastomoses were angiographically identified after n-BCA embolization (Figs. 4, 5, 6).
A 46-year-old man with metastatic neuroendocrine carcinoma revealed progression after partial hepatectomy and systemic chemotherapies. A Angiography following coil embolization of the gastroduodenal artery (GDA), posterior superior pancreaticoduodenal artery (PSPDA), and anterior superior pancreaticoduodenal artery (ASPDA) demonstrated avid filling of a supraduodenal artery (SDA; arrow) originating from a segmental right hepatic artery. B The SDA was selected using a 2.4F microcatheter, and angiography demonstrated the complex network supplying the distribution of the embolized PSPDA with parenchymal enhancement of the duodenum. C Postembolization angiography revealed subtle subtraction artifact of the n-butyl cyanoacrylate (n-BCA) cast and confirmed complete skeletonization. A 2.02 GBq resin microsphere whole liver-remnant dose was administered without complications
A 63-year-old man with metastatic colon cancer refractory to systemic therapies underwent skeletonization with coil embolization of the gastroduodenal artery (GDA) and right gastric artery (RGA) and consolidation of inflow by coil embolization of the accessory left hepatic artery (LHA). A Angiography demonstrated 2 supraduodenal arteries (SDAs) arising from the right hepatic artery (RHA) proximally (arrowheads) and distally (arrow). B The proximal SDA was better depicted by lobar angiography, and then C selected and injected, demonstrating parenchymal enhancement of the duodenum and communication with the distal SDA (arrow). D Cone beam C-arm computed tomography (CT) with contrast medium injected into the common hepatic artery (CHA) confirmed enhancement of the superior duodenum (arrow). E Cone beam C-arm CT after embolization revealed glue cast in vessels supplying the duodenum. F Angiography of CHA revealed subtraction artifact of the glue cast and no residual extrahepatic flow before administration of 1.57 GBq and 0.51 GBq resin microspheres to the RHA and LHA
A 69-year-old man with metastatic rectal carcinoma and previous left lobectomy underwent skeletonization with coil embolization of the gastroduodenal artery (GDA) and right gastric artery (RGA), 2 posterior superior pancreaticoduodenal arteries (PSPDA), and 2 supraduodenal arteries (SDAs). A Angiography 8 days later before administration revealed interval appearance of a new SDA (arrow). B Selective angiography of the new SDA revealed supply to the pancreas and duodenum, as well as contiguity with the segment 4 remnant artery. Coil embolization of this new SDA may have allowed continued perfusion of the arcade via the segment 4 remnant artery. C After glue embolization, the hepatoenteric communication was eradicated
Microspheres were administered proximal to the n-BCA embolized vessel in 5 patients (50 %). In one case (Fig. 2), because of only partial success of eliminating hepatofugal filling of the perihilar plexus by n-BCA embolization, a Surefire Infusion System catheter (Surefire Medical Inc., Westminster, CO) was deployed in the PHA distal to the persistent vessel for administration of RE microspheres. The Surefire Infusion System is an embolization-specific infusion catheter with an expandable conical mesh tip designed to eliminate reflux during infusion. Microspheres were administered distally in the other 4 patients.
One patient had accidental nontarget embolization of a middle hepatic artery (MHA) with n-BCA glue as a result of excessive volume administered and reflux, requiring a minor change of treatment plan. Originally, this patient was to undergo flow redistribution by coil embolization of an accessory LHA, followed by whole liver treatment via middle hepatic and replaced right hepatic arteries. Because of the nontarget n-BCA embolization, the administration was actually delivered via the accessory left and replaced right hepatic arteries. Another patient developed a duodenal ulcer 14 months after RE. Biopsy did not reveal microspheres. No RE-related gastrointestinal ulcerative complications were identified. In addition, no biliary or ischemic complications related to n-BCA were identified.
Discussion
Reported rates of gastrointestinal ulceration secondary to nontarget deposition of Y-90 microspheres vary widely in the literature between 0 and 29 % [2, 9]. In modern practices, rates of <5 % are achieved through the use of meticulous angiography, prophylactic embolization of hepatoenteric anastomoses, and distal administration of microspheres [9–12].
Even with state-of-the-art technology, though, the risk of nontarget RE remains. One recognized route of nontarget RE is via the hepatic hilar peribiliary plexus [8]. This network may include numerous vessels smaller than the limits of angiographic spatial resolution. In addition, the progressive increase in intrahepatic arterial resistance during administration of embolic Y-90 microspheres may result in reversal of flow in vessels that originally had hepatopetal flow and thus escaped detection at the time of skeletonization [7]. Elimination of hepatofugal flow into this network is necessary to ensure safety, but previously described methods of coil embolization or balloon occlusion have proven to be imperfect, perhaps because of the small vessel size and reversibility of flow in the network [7, 13–15].
The hepatic hilar peribiliary plexus is highly variable, and the anatomy literature describing it is inconsistent and contradictory. Individual named vessels within the plexus include the supraduodenal artery, retroportal artery, pancreaticoduodenal arteries, and retroduodenal artery (sometimes but not always equated with the posterior superior pancreaticoduodenal artery [16–18]). These vessels are identifiable and form complex collateral pathways in a high percentage of subjects. For instance, the supraduodenal artery, which is present in nearly all subjects, provides collateralization to neighboring vascular territories in nearly half [19]. Regardless of nomenclature, these arteries form complex and variable anastomoses with the cystic artery, RHA, LHA, PHA, and CHA, gastroduodenal artery (GDA), and superior mesenteric artery [16, 17, 19, 20].
Depiction of components of the network is possible by a number of different imaging modalities, aiding in their identification and elimination. Although digital subtraction angiography remains the mainstay modality for depiction of hepatic arterial anatomy and affords the highest spatial and temporal resolution, contrast-enhanced cone beam C-arm computed tomography and single photon emission computed tomography after injection of technetium-99 m-labeled macroaggregated albumin have each been demonstrated to have added utility in the direct or indirect identification of occult hepatoenteric anastomoses [6, 7, 9, 13, 21–23]. Neither is effective at identifying tiny peribiliary vessels, though, especially in the presence of nearby platinum coils and gas-filled viscera.
Even if these occult hepatoenteric anastomoses can be depicted, their small size and angulations present a clinical challenge. Standard 0.035-inch or 0.018-inch embolization coils may be inappropriately large or stiff, and even detachable coils and smaller caliber neurointerventional coils designed for small vessels may not be effective [24]. Vessel origins may be found in the distal lobar or segmental arterial tree, often with a sharp 180° takeoff, requiring the use of preshaped microcatheters and wires. In the present series, a J-tipped microcatheter was necessary for selection in 8 out of 10 cases, and still could not be advanced an adequate distance beyond the origin to provide a stable platform for the deployment of coils.
Although small vessel size and unstable catheter position may be indications for use of liquid embolics, even greater benefit may be seen when attempting to eliminate an entire network of vessels, as is frequently encountered near the hepatic hilum. Eliminating flow in a larger portion of the network not only abolishes flow from the catheterized feeding vessel, but also reduces the likelihood of further formation of collateral hepatoenteric vessels and reconstitution of major arteries such as the GDA or RGA [25]. In an analysis of hepatoenteric vessels apparent at the time of RE treatment after previous skeletonization, Abdelmaksoud found 13 arteries in 122 patients that were never apparent during the skeletonization angiogram [7]. Appearance of new collateral vessels was postulated to be due to interval hypertrophy or reversal of flow. Patients in whom collateral vessels developed remained at increased risk for ulceration even after these new vessels were coil embolized, postulated to be due to especially luxuriant hilar hepatoenteric networks and new collateral vessels too small to detect. Coil embolization of larger vessels such as the GDA may actually induce formation of small collateral vessels; the convention of preemptive embolization may be in need of reevaluation.
New collateral vessels originating distally, for instance supraduodenal arteries originating from the proximal right segmental arteries, are particularly problematic because the option of administration of RE microspheres distal to the vessel could result in incomplete treatment or could require an excessive number of fractionated administrations. Coil embolization of the parent artery with redistribution of supply from safer branches may be an option in some cases [26]. Although the use of liquid embolic materials carries a higher theoretical risk for bowel or biliary ischemia, judicious use has proven to be safe and effective for treatment of gastrointestinal hemorrhage [27, 28] and now also appears to be safe for hepatic arterial skeletonization. Other liquid embolic agents such as Onyx may prove to have their own advantages.
In conclusion, small complex hepatoenteric communications may be difficult to manage in RE patients. Complete skeletonization of the hepatic arterial tree may be facilitated by embolization with n-BCA glue. The use of n-BCA allows for more effective embolization of luxuriant complex perihilar networks that may not be as effectively occluded with coil embolization.
References
Sjoquist KM, Goldstein D, Bester L (2010) A serious complication of selected internal radiation therapy: case report and literature review. Oncologist 15:830–835
Naymagon S, Warner RRP, Patel KC et al (2010) Gastroduodenal ulceration associated with radioembolization for the treatment of hepatic tumors: an institutional experience and review of the literature. Dig Dis Sci 55:2450–2458
Ariel IM (1965) Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 Radiating Microspheres). Ann Surg 162:267–278
Simon N, Silverstone SM, Roach LC et al (1971) Intra-arterial irradiation of tumors: a safe procedure. Am J Roentgenol Radium Ther Nucl Med 112:732–739
Kennedy A, Nag S, Salem R et al (2007) Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the Radioembolization Brachytherapy Oncology Consortium. Int J Radiat Oncol Biol Phys 68:13–23
Riaz A, Lewandowski RJ, Kulik LM et al (2009) Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 20:1121–1130
Abdelmaksoud MHK, Hwang GL, Louie JD et al (2010) Development of new hepaticoenteric collateral pathways after hepatic arterial skeletonization in preparation for yttrium-90 radioembolization. J Vasc Interv Radiol 21:1385–1395
Michels NA (1953) Variational anatomy of the hepatic, cystic, and retroduodenal arteries: a statistical analysis of their origin, distribution, and relations to the biliary ducts in two hundred bodies. Arch Surg 66:20
Murthy R, Brown DB, Salem R et al (2007) Gastrointestinal complications associated with hepatic arterial yttrium-90 microsphere therapy. J Vasc Interv Radiol 18:553–561
Coldwell D, Sangro B, Wasan H et al (2011) General selection criteria of patients for radioembolization of liver tumors: an international working group report. Am J Clin Oncol 34:337–341
Nair J, Liu C, Caridi J et al (2010) Gastroduodenal ulcerations as a delayed complication of hepatic metastasis radioembolization. J Clin Oncol 28:e735–e736
Carretero C, Munoz-Navas M, Betes M et al (2007) Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol 102:1216–1220
Lewandowski RJ, Sato KT, Atassi B et al (2007) Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol 30:571–592
Salem R, Thurston KG (2006) Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol 17:1251–1278
Andrews JC, Walker SC, Ackermann RJ et al (1994) Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med 35:1637–1644
Michels NA (1951) The hepatic, cystic and retroduodenal arteries and their relations to the biliary ducts with samples of the entire celiacal blood supply. Ann Surg 133:503–524
Bertelli E, Di Gregorio F, Bertelli L et al (1996) The arterial blood supply of the pancreas: a review. II. The posterior superior pancreaticoduodenal artery. An anatomical and radiological study. Surg Radiol Anat 18:1–9
Northover JM, Terblanche J (1979) A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg 66:379–384
Bianchi H, Albanese E (1989) The supraduodenal artery. Surg Radiol Anat 11:37–40
Vellar ID (1999) The blood supply of the biliary ductal system and its relevance to vasculobiliary injuries following cholecystectomy. Aust N Z J Surg 69:816–820
Paprottka PM, Jakobs TF, Reiser MF et al (2012) Practical vascular anatomy in the preparation of radioembolization. Cardiovasc Intervent Radiol 35:454–462
Liu DM, Salem R, Bui JT et al (2005) Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol 16:911–935
Louie JD, Kothary N, Kuo WT et al (2009) Incorporating cone-beam CT into the treatment planning for yttrium-90 radioembolization. J Vasc Interv Radiol 20:606–613
Nambiar AP, Bozlar U, Angle JF et al (2008) Initial clinical experience with biopolymer-coated detachable coils (HydroCoil) in peripheral embolization procedures. J Vasc Interv Radiol 19:995–1001
Meer AB, Louie JD, Abdelmaksoud MHK et al (2011) Intrahepatic collateral supply to the previously embolized right gastric artery: a potential pitfall for nontarget radioembolization. J Vasc Interv Radiol 22:575–577
Bilbao JI, Garrastachu P, Herraiz MJ et al (2010) Safety and efficacy assessment of flow redistribution by occlusion of intrahepatic vessels prior to radioembolization in the treatment of liver tumors. Cardiovasc Intervent Radiol 33:523–531
Yonemitsu T, Kawai N, Sato M et al (2009) Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and N-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J Vasc Interv Radiol 20:1176–1187
Frodsham A, Berkmen T, Ananian C et al (2009) Initial experience using N-butyl cyanoacrylate for embolization of lower gastrointestinal hemorrhage. J Vasc Interv Radiol 20:1312–1319
Conflict of interest
Daniel Sze is a consultant to Abbott Vascular, Inc., a speaker for W. L. Gore, Inc., and a scientific/medical advisory board member of Jennerex Biotherapeutics, Inc., Treus Medical, Inc., RadGuard Medical, Inc., and SureFire Medical, Inc. Shaun Samuelson and John Louie have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samuelson, S.D., Louie, J.D. & Sze, D.Y. N-butyl Cyanoacrylate Glue Embolization of Arterial Networks to Facilitate Hepatic Arterial Skeletonization before Radioembolization. Cardiovasc Intervent Radiol 36, 690–698 (2013). https://doi.org/10.1007/s00270-012-0490-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-012-0490-1