Abstract
Purpose
A rare but described risk factor for deep venous thrombosis (DVT), predominately in the young, is congenital agenesis or atresia of the inferior vena cava (IVC). The optimal management for DVT in this subset of patients is unknown. We evaluated the efficacy of pharmacomechanical catheter-directed thrombolysis (PCDT) followed by systemic anticoagulation in the treatment of acute lower-extremity DVT in the setting of congenital IVC agenesis or atresia.
Materials and Methods
Between November of 2005 and May of 2010, six patients (three women [average age 21 years]) were referred to our department with acute lower-extremity DVT and subsequently found to have IVC agenesis or atresia on magnetic resonance imaging. A standardized technique for PCDT (the Angiojet Rheolytic Thrombectomy System followed by the EKOS Microsonic Accelerated Thrombolysis System) was used for all subjects. Successful thrombolysis was followed by systemic heparinization with transition to Coumadin or low molecular-weight heparin and compression stockings. Subjects were followed-up at 1, 3, and then every 6 months after the procedure with clinical assessment and bilateral lower-extremity venous ultrasound.
Results
All PCDT procedures were technically successful. No venous stenting or angioplasty was performed. The average thrombolysis time was 28.6 h (range 12–72). Two patients experienced heparin-induced thrombocytopenia, and one patient developed a self-limited knee hemarthrosis, No patients were lost to follow-up. The average length of follow-up was 25.8 ± 20.2 months (range 3.8–54.8). No incidence of recurrent DVT was identified. There were no manifestations of postthrombotic syndrome.
Conclusions
PCDT followed by systemic anticoagulation and the use of compression stockings appears to be safe and effective in relatively long-term follow-up treatment of patients who present with acute DVT and IVC agenesis or atresia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital inferior vena cava (IVC) agenesis or atresia is a rare risk factor for lower-extremity deep vein thrombosis (DVT) [1, 2]. Patients with IVC agenesis or atresia are typically asymptomatic owing to the development of extensive collaterals, which supplant the normal venous drainage from the lower extremities of the IVC. However, IVC agenesis or atresia may first present during evaluation of a new lower-extremity DVT and is predominantly seen in younger patients [3, 4].
Postthrombotic syndrome (PTS) is a common long-term complication of iliofemoral DVT. PTS may result in chronic pain, venous ulcers, work disability, major quality-of-life impairment, and economic costs, and it may have an even greater incidence in younger patients [5, 6]. Pharmacomechanical catheter-directed thrombolysis (PCDT) has been promising in decreasing the incidence of PTS after iliofemoral DVT compared with conventional anticoagulation [7]. However, reports of PCDT for iliofemoral DVT in the setting of IVC agenesis or atresia, including optimal technique, efficacy, and outcomes, are limited [8]. We describe our technique for PCDT, postprocedure management, and outcomes in a cohort of patients with IVC agenesis or atresia who presented with lower-extremity iliofemoral DVT.
Materials and Methods
Patient Selection and Study Design
This retrospective study was conducted with the approval of our Institutional Review Board, with waiver of informed consent, and was compliant with Health Insurance Portability and Accountability Act regulations. Between November of 2005 and May of 2010, six patients with acute lower-extremity DVT were referred to our division for PCDT; on further noninvasive evaluation of the patients, IVC agenesis or atresia was discovered. For each patient, the hospital electronic medical record and radiological images and reports were reviewed. Initial diagnosis of acute DVT was made with ultrasound, including Doppler examinations, in each patient. The diagnosis of IVC agenesis or atresia was made and/or confirmed in all patients as identified by lack or atresia of the IVC and presence of large paravertebral and lumbar collateral veins (Fig. 1) on magnetic resonance venography (MRV).
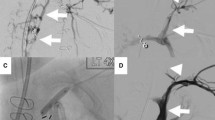
Axial (A) and coronal (B) postcontrast T1-weighted images with fat-saturation images show marked enlargement of paravertebral (solid arrows) veins, which have supplanted from the IVC the normal venous drainage of the lower extremities. The infrarenal IVC is not visualized in its expected location (dashed arrow), which given the well-developed paravertebral collateral veins, indicates congenital agenesis or atresia of the IVC. These findings correlate with the subsequent diagnostic venography (C)
PCDT Technique
A standardized technique for PCDT was used for all patients. Each patient was placed under intravenous conscious sedation with continuous hemodynamic and respiratory monitoring during the procedure. Using ultrasound guidance and with the patient in prone position, access to the popliteal vein of the affected lower extremity was obtained using a 5F micropuncture set. In cases of bilateral DVT, both popliteal veins were accessed simultaneously. A 0.035-inch glidewire was then inserted, and a 6F × 10-cm vascular sheath was placed. Initial venogram was performed through the sheath. Subsequently, the 6F AngioJet Rheolytic Thrombectomy System (DVX; Possis Medical, Minneapolis, MN) was placed over the wire and used in power pulse mode with a mixture of 15 mg tissue plasminogen activator (tPA; Genetech, San Francisco, CA) in a volume of 50 cc normal saline. The wire and AngioJet system were placed as cephalad as possible in the iliac vein (Fig. 2). The mixture was infused proximally to distally, allowed to dwell for approximately 15 min, and subsequently aspirated using the AngioJet system.
Fluoroscopic spot film image from diagnostic venography from a popliteal approach and with the patient prone shows the wire (arrow) coiled in the cephalad aspect of the left iliac vein, where it joins enlarged lumbar veins because an IVC is not present. The subsequent PCDT was performed with the catheter extending up to this point, and the overnight thrombolysis catheter was also placed with its tip in this cephalad portion of the left iliac vein. Nonenlarged abdominal wall collaterals (arrowheads) are identified, indicating the acute aspect of the occlusion of the venous outflow of the lower extremity and reinforcing the diagnosis of congenital IVC agenesis or atresia
Repeat venogram was performed followed by placement of a thrombolysis catheter (EKOS Microsonic Accelerated Thrombolysis System; EKOS, Bothell, WA) through the affected area for overnight thrombolysis. Again, the catheter was placed with the tip as cephalad as possible in the iliac vein. TPA was infused at 0.5–1 mg/h in each catheter, and fibrinogen levels were checked every 6 h. Low levels of unfractionated heparin were infused through the sheath to a total of 200–500 u/h. In patients with bilateral DVT, this was performed in both lower extremities simultaneously. The patient was then brought back for venography within 12–24 h after the initial procedure. If it was determined on repeat venography that there was still significant thrombus burden, the thrombolysis was continued for another 12–24 h. After complete or nearly complete thrombus resolution, the catheters were removed, and the subject was systemically heparinized with goal PTT of 60–80.
Posttreatment Therapy and Follow-Up
During the hospital stay, the patients were transitioned from intravenous heparin to either Coumadin (Warfarin Sodium Tablets, USP; Bristol-Myers Squibb, New York, NY) with a target INR of 2.0–2.5 or placed on intramuscular lower molecular-weight heparin (Fragmin, Pfizer, New York, NY; Lovenox, Sanofi-Aventis, Bridgewater, NJ). Subjects were then maintained on Coumadin or low molecular-weight heparin as outpatients. In those patients whose d-dimer normalized and who were at least 1 year out from the acute episode of DVT, anticoagulation discontinuation was considered. In those patients with persistently increased d-dimer levels or who were found to have an underlying hypercoagulability condition, anticoagulation was extended [9].
On discharge from the hospital, patients were strongly encouraged to wear thigh-high bilateral graded-compression stockings (20–30 mm Hg). Subjects were followed-up at 1, 3, and then every 6 months after the procedure with clinical assessment and bilateral lower-extremity ultrasound at each visit.
Data Collection
Data collection was performed using the electronic medical records, including review of clinical notes, laboratory values, procedure images, and procedure reports. The cross-sectional imaging (MRV) studies and reports were also reviewed. Additional information collected included patient demographics, presenting thrombosis site and extent, presenting contributing factors, MRV findings and extent of IVC atresia, duration of PCDT, technical success of PCDT, and periprocedural complications.
Technical success of the procedure was characterized in accordance with previously established reporting standards. This was assessed on the final procedural venogram with estimation of the degree of thrombolysis: grade I = <50% thrombus removal; grade II = 50–95% thrombus removal, or grade III = 95–100% thrombus removal. Technical success was determined as grade II or III (>50% thrombus removal) thrombolysis [10]. Incidence of recurrent DVT, Venous Clinical Severity Scores (VCSS), and clinical, etiologic, anatomic, and pathophysiologic (CEAP) classification [11] were noted in follow-up visits.
Results
Six patients (three men and three women; average age 21 ± 5 years [range 15–30]) were included in the study (Table 1). On presentation to our division with acute lower-extremity DVT diagnosed by ultrasound, none of our patients had diagnosis of IVC agenesis or atresia. Three patients had bilateral lower-extremity DVT; one patient had unilateral right lower-extremity DVT; and the other two patients had unilateral left lower-extremity DVT.
Each of the three patients with bilateral lower-extremity DVT and one of the patients with unilateral left DVT underwent MRV of the abdomen and pelvis during further workup for the DVT identified on ultrasound before PCDT. The other two patients underwent initial venography and attempted catheterization of the IVC, at which point an IVC anomaly was suspected. These two patients then underwent MRV of the abdomen and pelvis, and a diagnosis of IVC agenesis or atresia was made. Five patients (5 of 6, 83%) were found to have relative risk factors for DVT, including recent oral contraceptive pill use and immobilization secondary to lower-extremity trauma. Two patients were subsequently found to have risk factors for hypercoagulability (Table 1).
All PCDT procedures were technically successful. In all patients, venous drainage was through lumbar collaterals in the abdomen on final venography. The average thrombolysis time was 28.6 ± 21.7 h (range 12–72). Two patients experienced heparin-induced thrombocytopenia (HIT) during treatment, and a nonheparin anticoagulation medication (Argatroban; GlaxoSmithKline, Brentford, UK) was subsequently used in these patients. One patient was clinically suspected to have HIT due to identification of rethrombosis on 48 h follow-up venography during PCDT. The second patient was clinically suspected to have HIT 3 days after the PCDT was finished due to thrombocytopenia. HIT was confirmed with positive results from heparin-PF4 antibodies assay in both patients. One patient developed a knee hemarthrosis, which did not require intervention; this was noted as a minor complication. There were no major procedural complications, including pulmonary embolism or major bleeding.
The average length of follow-up was 25.8 ± 20.2 months (range 3.8–54.8); no patient was lost to follow-up. Anticoagulation had been discontinued in two patients at 26 and 16 months after the initial episode of DVT. The other four remained on anticoagulation (warfarin, fragmin, and lovenox (two patients)). All patients had normal VCSS and CEAP classifications, and there were no incidence of recurrent DVT or findings to suggest PTS.
Discussion
The optimal management of young patients who present with DVT and are discovered to have IVC absence or atresia is unclear due to its relative rarity, and given the small patient population in our study, more experience will be needed. However, PCDT, followed by systemic anticoagulation and the use of compression stockings, appears to be safe and effective in relatively long-term follow-up treatment of patients who present with acute DVT and IVC agenesis or atresia.
There have been case reports with little long-term patency data (approximately 6 months) describing endovascular recanalization and stenting of congenital IVC atresia [12], similar to what has been advocated in cases of acquired IVC occlusion [13, 14]. However, the pathophysiology and subsequent venous drainage that develops for the lower extremities between these two entities are quite different. Patients with IVC anomalies typically develop robust collateral circulation through lumbar and paravertebral collaterals, with or without hemiazygos and azygos continuation of the IVC. Although the relative caval stasis may predispose to DVT, many patients with IVC atresia likely never experience DVT unless additional risk factors for development of DVT exist, as seen in five of our six patients.
Given the extensive venous collaterals that patients with IVC atresia develop, we have adopted an alternative approach to more invasive treatments, such as recanalization and stenting, with optimization of the inherent venous drainage patterns present and rapid restoration of the iliofemoral venous outflow. A similar case series to ours has also been recently reported in the literature [8] with similar results.
Given these results, young patients who present with extensive thrombosis (bilateral), or who present with lower-extremity thrombosis that extends into the iliac veins, may benefit from initial cross-sectional imaging to identify the extent of the thrombosis and concurrently evaluate the IVC. Each of the patients in our series was identified to have well-developed lumbar and paravertebral collaterals on venography and MRV (Fig. 1), thus replacing the natural venous return to the heart. We also did not identify any enlarged abdominal wall collaterals in any of our patients as often seen in patients with acquired IVC occlusion (Fig. 2). The strength of our study is the relatively standardized treatment that we instituted in our patients and the long-term follow-up we had (average follow-up >2 years).
The IVC is created by the fusion of three sets of paired veins, specifically the posterior cardinal, subcardinal, and supracardinal veins, during weeks 6–8 of embryonic development. It is the failure of these paired veins to fuse into a unilateral right-sided venous system that leads to an anomalous IVC. Anomalous development of the IVC has been well documented: The estimated prevalence of complete absence of the IVC is 0.3–0.5% of otherwise healthy individuals. The prevalence may increase ≤2% in patients with other cardiovascular defects [15, 16].
Lower-extremity DVT is multifactorial in etiology and is often associated with Virchow’s triad, which includes alterations in blood flow (stasis), injury to the vascular endothelium, and abnormalities in the constitution of blood (hypercoagulability). Lifetime incidence of DVT is approximately 0.1% and even more rare in patients <30 years of age [17, 18]. Secondary to venous stasis, congenital absence or atresia of the IVC is a risk factor for DVT in the iliac and femoral veins. According to previous studies, ≤5% of patients with iliofemoral DVT have a congenital caval defect [2, 3, 19].
PTS, which results in chronic pain, venous ulcers, work disability, major quality-of-life impairment, and economic costs, is a common complication of DVT, and its incidence among teenagers and young adults may approach 60% [5, 6, 9]. PCDT has the ability to prevent PTS by restoring venous patency and preserving valvular function, thereby decreasing the risks of pulmonary embolism and recurrent DVT and providing immediate symptom relief that may otherwise take days to weeks to resolve with anticoagulation alone. In young patients, such as those who present with DVT and who are found to have IVC absence or atresia, using PCDT successfully in DVT has the potential to avoid decades of morbidity.
Length of anticoagulation for these patients is not clear, and some investigators have suggested life-long continuation [20, 21]. We continue anticoagulation until it is identified that the d-dimer normalizes, reflecting a return to a nonthrombogenic state [9]. At this point, we discontinue anticoagulation and have not had an incident of recurrent DVT. Anomalies of the IVC have been linked to hypercoagulability disorders, such as factor V Leiden, prothrombin gene mutation, low protein S levels, high homocysteine concentration, methylenetetrahydrofolate reductase gene mutation, and antiphospholipid antibodies [22–24], as was seen in two of our six patients (33%). In these patients with inherent hypercoagulability, we recommend life-long anticoagulation. We also recommend lifetime use of graded compression stockings in all patients, a low-cost and likely highly beneficial intervention.
Limitations
Aside from the small sample size, our study is limited by a single treatment arm. We are also limited in that we have no temporal or pathologic confirmation that each patient had in utero IVC atresia, although we had no history to indicate that these were cases of acquitted IVC occlusion.
Our study is also limited by a single treatment algorithm. Currently numerous devices for PCDT are available, which can be broadly divided into those designed for passive or assisted infusion of fibrinolytic drugs and those for percutaneous mechanical thrombectomy, including clot disintegration and aspiration. In our study, PCDT was performed with a combination of the AngioJet system in the power-pulse mode followed by the EKOS system.
We chose to start with the AngioJet system in the power-pulse mode combined with tPA in an attempt to initially lyse and removed the bulk of the thrombus and create venous channels within the thrombus for subsequent thrombolysis. The intent is to augment the efficacy of subsequent thrombolysis and possibly shorten procedure time and necessary thrombolytic drug required. We then followed with extended thrombolysis using the EKOS drug-delivery catheters. These catheters emit high-frequency, low-power microsonic energy, which helps temporarily loosen and separate the fibrin for more blood clot permeability. Loosened fibrins increase the availability of more plasminogen activation receptor sites, and the microsonic energy helps drive the thrombolytic agents deep into the blood clot to accelerate the thrombolysis.
These are two devices and systems that we use routinely in our practice of PCDT; however, we recognize there are other devices, thrombolysis catheters, and treatment algorithms for PCDT in use that may be of comparable efficacy. In addition, retrievable IVC filter placement is advocated by some for thrombosed lower-extremity or IVC PCDT; however, given the lack of a normal IVC as a possible conduit for clinically significant pulmonary emboli in this patient population, we surmised no benefit in their use.
Conclusion
In conclusion, given the findings in our cohort of patients, PCDT followed by systemic anticoagulation and use of compression stockings, appears to be safe and effective in relative long-term follow-up treatment of patients who present with acute DVT and IVC agenesis or atresia.
References
Shah NL, Shanley CJ, Prince MR et al (1996) Deep venous thrombosis complicating a congenital absence of the inferior vena cava. Surgery 120(5):891–896
Ruggeri M, Tosetto A, Castaman G et al (2001) Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosis. Lancet 357(9254):441
Chee YL, Culligan DJ, Watson HG (2001) Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 114(4):878–880
Obernosterer A, Aschauer M, Schnedl W et al (2002) Anomalies of the inferior vena cava in patients with iliac venous thrombosis. Ann Intern Med 136(1):37–41
Vedantham S (2011) Preventing pediatric postthrombotic syndrome: preparing the way. J Vasc Interv Radiol 22(3):405–407
Kuhle S, Koloshuk B, Marzinotto V et al (2003) A cross-sectional study evaluating post-thrombotic syndrome in children. Thromb Res 111(4–5):227–233
Goldenberg NA, Durham JD, Knapp-Clevenger R et al (2007) A thrombolytic regimen for high-risk deep venous thrombosis may substantially decrease the risk of postthrombotic syndrome in children. Blood 110(1):45–53
Broholm R, Jorgensen M, Just S et al (2011) Acute iliofemoral venous thrombosis in patients with atresia of the inferior vena cava can be treated successfully with catheter-directed thrombolysis. J Vasc Interv Radiol 22(6):801–805
Goldenberg NA, Knapp-Clevenger R, Manco-Johnson MJ (2004) Elevated plasma factor VIII and d-dimer levels as predictors of poor outcomes of thrombosis in children. N Engl J Med 351(11):1081–1088
Vedantham S, Grassi CJ, Ferral H et al (2009) Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol 20(Suppl 7):S391–S408
Eklof B, Rutherford RB, Bergan JJ et al (2004) Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 40(6):1248–1252
Porter D, Rundback JH, Miller S (2010) Sharp recanalization using a subintimal reentry device, angioplasty, and stent placement for severely symptomatic iliofemoral deep venous thrombosis secondary to congenital aplasia of the inferior vena cava. J Vasc Interv Radiol 21(11):1765–1769
Robbins MR, Assi Z, Comerota AJ (2005) Endovascular stenting to treat chronic long-segment inferior vena cava occlusion. J Vasc Surg 41(1):136–140
Razavi MK, Hansch EC, Kee ST et al (2000) Chronically occluded inferior venae cavae: endovascular treatment. Radiology 214(1):133–138
Anderson RC, Adams P Jr, Burke B (1961) Anomalous inferior vena cava with azygos continuation (infrahepatic interruption of the inferior vena cava). Report of 15 new cases. J Pediatr 59:370–383
Chuang VP, Mena CE, Hoskins PA (1974) Congenital anomalies of the inferior vena cava. Review of embryogenesis and presentation of a simplified classification. Br J Radiol 47(556):206–213
Nordstrom M, Lindblad B, Bergqvist D et al (1992) A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med 232(2):155–160
Anderson FA Jr, Wheeler HB, Goldberg RJ et al (1991) A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 151(5):933–938
Timmers GJ, Falke TH, Rauwerda JA et al (1999) Deep vein thrombosis as a presenting symptom of congenital interruption of the inferior vena cava. Int J Clin Pract 53(1):75–76
Dean SM, Tytle TL (2006) Acute right lower extremity iliofemoral deep venous thrombosis secondary to an anomalous inferior vena cava: a report of two cases. Vasc Med 11(3):165–169
Tsuji Y, Inoue T, Murakami H et al (2001) Deep vein thrombosis caused by congenial interruption of the inferior vena cava—a case report. Angiology 52(10):721–725
Yun SS, Kim JI, Kim KH et al (2004) Deep venous thrombosis caused by congenital absence of inferior vena cava, combined with hyperhomocysteinemia. Ann Vasc Surg 18(1):124–129
Parma M, Belotti D, Marinoni S et al (2003) Congenital absence of the inferior vena cava and genetic coagulation abnormalities: a rare associated risk factor for recurrent idiopathic deep vein thrombosis. Clin Appl Thromb Hemost 9(4):347–348
Schneider JG, Eynatten MV, Dugi KA et al (2002) Recurrent deep venous thrombosis caused by congenital interruption of the inferior vena cava and heterozygous factor V Leiden mutation. J Intern Med 252(3):276–280
Conflict of interest
The authors declared that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganguli, S., Kalva, S., Oklu, R. et al. Efficacy of Lower-Extremity Venous Thrombolysis in the Setting of Congenital Absence or Atresia of the Inferior Vena Cava. Cardiovasc Intervent Radiol 35, 1053–1058 (2012). https://doi.org/10.1007/s00270-011-0247-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-011-0247-2