Abstract
Purpose
This study was designed to evaluate overall survival after radioembolization or best supportive care (BSC) in patients with chemotherapy-refractory liver-dominant metastatic colorectal cancer (mCRC).
Methods
This was a matched-pair comparison of patients who received radioembolization plus BSC or BSC alone for extensive liver disease. Twenty-nine patients who received radioembolization were retrospectively matched with a contemporary cohort of >500 patients who received BSC from 3 centers in Germany. Using clinical databases, patients were initially matched for prior treatments and tumor burden and then 29 patients were consecutively identified with two or more of four matching criteria: synchronous/metachronous metastases, tumor burden, increased ALP, and/or CEA >200 U/ml. Survival was calculated from date of progression before radioembolization or BSC by using Kaplan–Meier analysis.
Results
Of 29 patients in each study arm, 16 pairs (55.2%) matched for all four criteria, and 11 pairs (37.9%) matched three criteria. Patients in both groups had a similar performance status (Karnofsky index, median 80% [range, 60–100%]). Compared with BSC alone, radioembolization prolonged survival (median, 8.3 vs. 3.5 months; P < 0.001) with a hazard ratio of 0.3 (95% confidence interval, 0.16–0.55; P < 0.001) in a multivariate Cox proportional hazard model. Treatment-related adverse events following radioembolization included: grade 1–2 fatigue (n = 20, 69%), grade 1 abdominal pain/nausea (n = 14, 48.3%), and grade 2 gastrointestinal ulceration (n = 3, 10.3%). Three cases of grade 3 radiation-induced liver disease were symptomatically managed.
Conclusions
Radioembolization offers a promising addition to BSC in treatment-refractory patients for whom there are limited options. Survival was prolonged and adverse events were generally mild-to-moderate in nature and manageable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the second most common malignancy in Europe [1]. Many patients with CRC (approximately 15–25%) develop liver metastases [2]. Although new chemotherapy regimens have improved the control of liver metastases and rendered an increasing number of patients resectable, the recurrent nature continues to present a life-limiting prognosis [3–5]. Nevertheless, overall survival for unresectable CRC liver metastases have been extended beyond 2 years by using combinations, including oxaliplatin or irinotecan plus a fluoropyrimidine, and biologic agents, such as vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR) inhibitors [6, 7].
In parallel, locally ablative procedures, such as radiofrequency ablation (RFA), are increasingly considered beneficial for patients with unresectable liver-only disease who present with tumors ≤3–4 cm [8]. RFA may consolidate the treatment response with chemotherapy to increase the number of patients eligible for resection [9]. At our clinic, we have extensive experience with image-guided interstitial brachytherapy for patients ineligible for RFA due to the location (adjacent to liver hilum, common bile duct, or hepatic bifurcation) or the size of liver metastases (>5 cm) [10, 11].
One of the major challenges in advanced CRC today is the growing proportion of patients who have maintained good performance status but present with extensive (≥20%) liver involvement, having exhausted all other therapeutic options. In this context, the transarterial administration of radioactive yttrium-90 (90Y)-labeled resin microspheres (radioembolization) represents a promising approach [12–14]. Even at this late stage, improved disease control following radioembolization is highly predictive of prolonged survival compared with historic controls and can downsize tumors sufficiently to enable potentially curative resection or ablation [13–17]. For ethical reasons, it has not been possible to conduct a randomized, controlled clinical trial to compare radioembolization with best supportive care (BSC) in this setting without crossover, thereby confounding a fair comparison of the impact on overall survival. With the absence of clinical trials in this setting, we undertook an analysis of patients who were refractory to all recommended chemotherapy (after 2–6, median 3 lines of chemotherapy; Table 1) or who had refused further chemotherapy at the time of progression. Patients prospectively received radioembolization plus best supportive care and were matched with a parallel cohort of patients who received BSC only (which means best palliative care with the intent to maximize quality of life) from surrounding hospitals and clinics. Further chemotherapy or antineoplastic therapy was originally not intended due to patient’s refusal or exhausted options, although nearly a third (31%) of all patients were subsequently able or willing to receive chemotherapy following radioembolization.
Patients and Methods
Study Design
This was a matched-pair comparison of patients who received radioembolization plus BSC or BSC alone for chemotherapy-refractory, liver-dominant colorectal metastases in the salvage setting. Patients treated prospectively with radioembolization were retrospectively paired with controls who received BSC only. To achieve the best match, the clinical records of a cohort of more than 500 patients from 3 centers were evaluated: Universitätsklinikum Magdeburg (n = 348); Klinikum Magdeburg (n = 86); and Universitätsmedizin Berlin, Charité Campus Virchow (n = 120). Matching pairs were identified in two stages: initially matching for prior treatment history and tumor burden and subsequently, by the following four matching criteria: liver involvement (±20% absolute difference); synchronous versus metachronous metastases; alkaline phosphatase (ALP) increase versus no increase; and carcinoembryonic antigen (CEA) ≥200 ng/ml versus <200 ng/ml. The first 29 consecutive matching patients identified were included in this analysis.
The primary endpoint was overall survival (OS) from the date of progression of the liver before radioembolization or prior commencement of BSC assessed radiologically until further progression (after radioembolization or BSC), evaluated by radiological imaging or clinically. Patients who received radioembolization received radiological and clinical follow-up examinations; progression was evaluated mainly by radiological imaging. Control group patients were routinely seen in oncological settings, and progression (hepatic and/or extrahepatic) was evaluated at the discretion of the oncologist (imaging not mandatory). When there was a significant increase of tumor markers, weight loss, and decrease of the performance status of at least one point, progression was assumed. Secondary endpoints were safety and tolerability of radioembolization, progression-free survival (PFS), and overall response rate, by RECIST, from the date of radioembolization. Ethics approval was obtained to conduct these analyses.
Patients
All patients had liver-dominant mCRC and intrahepatic tumor progression as confirmed by imaging (computer tomography [CT]/magnetic resonance imaging [MRI]) as well as tumor markers (CEA) and/or clinical symptoms. Patients who received BSC received measures designed to provide palliation of symptoms and improve quality of life. Because the patients were refractory to all recommended chemotherapy or had refused further chemotherapy at the time of progression, further chemotherapy or antineoplastic therapy was not intended, although nearly a third of all patients were subsequently able or willing to receive chemotherapy after radioembolization.
Consecutive patients who received treatment from the multidisciplinary team at the Universitätsklinikum in Magdeburg were considered as candidates for radioembolization if they presented with extensive liver involvement (≥20% of total liver volume) and none or only nonprogressive extrahepatic deposits. Patients were only considered for radioembolization if they were progressive, ineligible for all other forms of tumor-directed therapy, and able to give informed consent [18].
Candidates for radioembolization were required to have: adequate renal function (creatinine <1.5× normal values or creatinine clearance >50 ml/min) and hemopoietic function (leucocytes >1,500/mm3; platelet count >100,000/mm3); sufficient liver function (defined as absence of ascites or synthetic liver dysfunction, together with total bilirubin <1.75 mg/dl [<30 μmol/L], and AST, ALT, and ALP each <4× upper limit of normal); hepatic arterial anatomy that would enable safe delivery of microspheres to the liver only; liver-to-lung shunting of <20% on a pretreatment technetium-99m labelled macro-aggregated-albumin [99mTc-MAA] nuclear scan; and a patent main portal vein.
Radioembolization Planning and Treatment
Treatment was typically a two-stage process involving extensive workup (to assess the appropriateness of the patient and prepare the liver for treatment) and then administration of the microspheres. A detailed account of our treatment protocol has been published previously [18]. All imaging studies were read by radiologists and nuclear medicine specialists, and the decision to treat was made by a tumor board that included medical and surgical oncologists.
Meticulous celiac and superior mesenteric angiography was undertaken to map the hepatic arterial tree, identify arterial feeders to the gastrointestinal tract, and coil embolize the gastroduodenal and right gastric arteries and any other gastrointestinal tract feeders. Once the hepatic arterial blood supply had been isolated, the 99mTc-MAA injection (Tc-99m-LyoMAA, Covidien, Neustadt/Donau, Germany) was delivered into the proper hepatic artery when whole liver radioembolization was performed and in the right and left hepatic artery separately when the treatment approach was sequential lobar without repetition before second radioembolization. Afterwards, a gamma camera (E.CAM 180, Siemens, Erlangen, Germany) determined the extent of hepatopulmonary shunting. A SPECT scan of the upper abdomen was performed, and if nontarget extrahepatic seeding of 99mTc-MAA was found, the intra-arterial angiography procedure was repeated and modified accordingly.
The activity of 90Y-resin microspheres (SIR-Spheres; Sirtex Medical Limited, Sydney, Australia) was calculated by the body surface area (BSA) method using the formula:
The activity was reduced if there was excessive liver-lung shunting (>10%). Up to 2 weeks later, 90Y-resin microspheres were delivered via a temporary transfemoral catheter into the proper hepatic artery as a single whole-liver administration or into the lobar arteries as a sequential treatment of each lobe 4–8 weeks apart. All patients were admitted the day before the procedure and typically discharged 2 days later.
Clinical Assessments
Hematologic, liver function and blood biochemistry tests, and physical examination were performed pretreatment and on day 1 postradioembolization. Patients were monitored by MRI scan or abdomen-pelvis CT and any changes in both intra- and extrahepatic lesions were assessed at week 6 and every 3 months thereafter until disease progression. Tumor response to radioembolization was evaluated by consensus of two radiologists using RECIST. Throughout the follow-up period, physical examinations and serum liver function tests were performed at regular intervals, and adverse events were assessed and recorded by using CTCAE v3.0.
In the control cohort, the “time of progression” after the last chemotherapy and clinical symptoms, changes in blood test results, or radiological imaging consistent with further disease progression were recorded for inclusion in this analysis.
Statistical Analysis
Descriptive statistics were calculated for quantitative variables; frequency counts by category were calculated for qualitative variables; 95% confidence intervals [CI] are presented as appropriate. P values were considered significant if <0.05. Treatment and control groups were compared regarding baseline characteristics; continuous baseline characteristics were evaluated by using one-way ANOVA; dichotomous variables were evaluated by using Fisher’s exact test; and ordinal categorical data were assessed by using the Cochran–Mantel–Haenszel row mean score statistic with modified ridit scores.
The primary study endpoint was overall survival, which was evaluated by using Kaplan–Meier analysis and compared between groups by a log-rank test. The hazard ratios (HR) for baseline covariates (including Karnofsky-Index, tumor load, number of lines of chemotherapy, and response to EGFR inhibitor therapy as measured by acne-like skin rash [19]) were estimated for overall survival by univariate Cox proportional-hazards model (SAS, Cary, NC). A stepwise multivariate Cox proportional-hazards model utilizing a backwards elimination procedure was then constructed to assess the joint contribution of baseline parameters to prediction of overall survival.
Results
Patients
Fifty-eight patients (29 radioembolization and 29 BSC), well-matched for all baseline parameters, treated as salvage patients between June 2005 and March 2008, were included in this analysis. More than half (16 pairs; 55.2%) of radioembolization and control patients matched on all four predefined matching criteria; 11 pairs (37.9%) matched on three and 2 pairs (6.9%) on two criteria. There was no difference in performance status between the groups’ Karnofsky Index: median 80% (range 60–100%; Table 1).
All patients presented with extensive liver tumor involvement: median 30% of whole liver volume (range 20–50%) for the radioembolization group and 25% (range 10–75%) for the BSC group. Approximately half of patients, regardless of treatment group, had limited concomitant extrahepatic disease. The disease course appeared to be similar between the groups with a mean (±SD) time between initial diagnosis of CRC and confirmation of metastases of 6.1 (±10.7) months. Approximately 60% of patients in both groups had synchronous metastases.
Each study group was well matched for treatment history; a high proportion of patients in each group had received bevacizumab (51.7%; 48.3%) and/or cetuximab (51.7%; 65.5%), respectively.
Radioembolization Treatment and Response by RECIST
Planar scintigraphy showed no dose-relevant shunting of 99mTc-MAA to the lung. A median activity of 1.76 gigabequerels [GBq] (mean, 1.71; range, 0.93–2.56 GBq) of 90Y-resin microspheres was administered per patient. Sixteen patients (55.2%) received a single whole-liver administration, six patients (20.7%) sequential lobar treatments, two patients (6.9%) were treated in the right lobe, and two patients (6.9%) in the left lobe only. Three patients (10.3%) received whole-liver administration and repeated treatment of the left lobe.
Nearly a third of patients (31%) treated with radioembolization were subsequently able or willing to receive further systemic chemotherapy (median 1 line, range, 1–2) comprising fluoropyrimidine (9 patients), cetuximab (n = 5), irinotecan (n = 4), oxaliplatin (n = 1), and/or mitomycin C (n = 1).
After radioembolization, a partial response was observed in 12 patients (41.4%) and stable disease in 5 patients (17.2%). Eleven patients (37.9%) had progressive disease, and the response in one patient (3.4%) could not be evaluated because of early death after radioembolization due to cerebral stroke (5 weeks postintervention). The median progression-free survival (PFS) in patients who received radioembolization was 5.5 months. The median PFS in those who received BSC was 2.1 months, where progression was defined as a clinically significant change in symptoms or CEA levels, or confirmed by radiological imaging. PFS indicates the length of time after inclusion in our study (either in radioembolization or in BSC group) until progression. It indicates the interval between “progression” and “further progression.”
Overall Survival
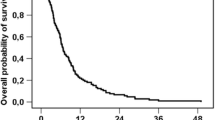
Patients who received radioembolization plus BSC survived significantly longer than the control cohort who received only BSC (median overall survival: 8.3 vs. 3.5 months; hazards ratio (HR), 0.26; 95% confidence interval (CI), 0.15–0.48; P < 0.001). This benefit was clearly evident at 3 months (97% vs. 59% survival) and sustained through the 12-month follow-up (24% vs. 0% survival; Fig. 1).
Radioembolization and Karnofsky Index reduced the risk of death based on the univariate Cox proportional hazards model for overall survival (HR, 0.26; 95% CI, 0.15–0.48; P < 0.001; and HR, 0.97; 95% CI, 0.95–1; P = 0.038, respectively; Table 2). In the multivariate analysis, radioembolization was the only significant predictor for prolonged survival (HR, 0.3; 95% CI, 0.16–0.55; P < 0.001), whereas the extent of liver involvement was associated with an increased risk of death (HR, 1.03; 95% CI, 1.0–1.06; P = 0.028).
Adverse Events
Adverse events after radioembolization were predominately transient and self-limiting. These included grade 1–2 fatigue in 20 patients (69%) in the first 14 days postradioembolization and was attributed in all cases as probably related to treatment. Mild abdominal pain and nausea (grade 1) occurred during the first 24 h postradioembolization in 14 patients (48.3%). Three patients (10.3%) developed a grade 2 gastrointestinal ulcer. All gastrointestinal events were attributed as definitely related to radioembolization. All patients were managed medically and were not considered life-threatening.
Radioembolization-induced liver disease (REILD) presents with ascites, nonelevated liver enzymes (except for ALP and GGPT), and a significant bilirubin increase [20]. Three cases of grade 3 REILD were treated symptomatically and medically managed and were not considered life-threatening (median survival, 9.8 months; range, 9–16.6).
Discussion
Radioembolization in addition to BSC provides substantial clinical benefit as evidenced by significantly prolonged overall survival compared with BSC alone in a well-matched cohort of patients with extensive, liver-dominant refractory disease for whom there are limited treatment options. In a contemporary treatment setting, liver-directed treatment with 90Y-resin microspheres was the most significant independent predictor for prolonged overall survival on multivariate analysis. Meticulous pretreatment planning and careful patient selection ensured that radioembolization was well-tolerated, and all three cases of REILD were symptomatically managed and not considered life-threatening.
Forty-one percent of our patients showed partial response, and 38% showed progressive disease after radioembolization according to RECIST criteria. However, the imaging evaluation regarding response after radioembolization is difficult by CT or MRI. Traditional methods have been size reduction on CT through the application of WHO or RECIST criteria, which present some pitfalls when used for evaluation after radioembolization. Response seen on imaging after radioembolization may be present as necrosis, edema, and peritumoral hemorrhage, which might result in an increase in tumor size. Using RECIST-criteria, this may represent progressive disease. Recommendations for tumor response after ablative therapies should include tumor necrosis and lack of enhancement but well-defined imaging criteria based on response following this treatment need to be established.
The median survival of 8.3 months with radioembolization (after a median of three lines of chemotherapy) is consistent with results from similar cohorts of chemotherapy-refractory patients with CRC liver-predominant metastases [13–17, 21]. In these studies, median survivals were between 9.9 months (n = 44)19 and 12.6 months (n = 50)14 after radioembolization. Comparisons with other innovative liver-directed treatments, such as stereotactic body radiation therapy, recently reported in the literature, are more difficult because of the limited extent of liver disease as well as the undefined nature and extent of prior systemic chemotherapy in these studies [22]. However, overall survivals with radioembolization compare favorably with recent studies in mCRC using new biological agents where median overall survival were 6.1 months with cetuximab versus 4.6 months with BSC (after 80% of patients had received ≥3 lines of chemotherapy) and 6.4 months with panitumumab versus similar survival with BSC followed by crossover to panitumumab at progression (after ≥2 prior lines of chemotherapy) [23, 24].
Key questions remain about the role of radioembolization for patients with chemorefractory CRC liver-dominant disease: Should the survival benefits reported in this and other prospective trials be confirmed in further randomized trial(s) against placebo, BSC, or other agents of limited efficacy? This raises a question of whether it is ethical to randomize a patient with an imminently terminal prognosis to BSC when a potentially life-extending therapy is available. This trial compared overall survival after radioembolization with matched patients who received BSC, whereas the randomized, controlled trial conducted by Hendlisz and colleagues enabled control patients to crossover to radioembolization upon disease progression [21]. Both studies were adequately powered and demonstrated that radioembolization using 90Y-resin microspheres resulted in a significant extension in progression-free survival or time to progression, which were remarkably consistent. Moreover, overall survival was significantly prolonged in the current trial and radioembolization was the only factor significantly associated with prolonged survival in the multivariate analysis. The evidence suggests that radioembolization should be considered as a treatment option for patients with liver-only or liver-dominant CRC-metastases who have failed or are intolerant of chemotherapy.
The key limitation of our study is the small number of patients and the retrospective nature of the design. Patients in the control group were not given the opportunity to receive radioembolization nor were they given the opportunity to refuse treatment; therefore, the control group may include patients who were less willing or able to receive additional treatment. This is an acknowledged weakness of retrospective studies in this clinical setting, although it is our contention that this would have limited impact on the findings of our study, because the patients in each arm had very similar performance status.
Candidates for radioembolization were selected only if they had extensive liver involvement (>20%) and were ineligible for other local ablative or systemic therapies. As the course of mCRC progresses, more patients are likely to be excluded from treatment using radioembolization due to the development of life-limiting extrahepatic metastases, excessive hepatic tumor burden, and/or compromised residual liver function. Therefore, using radioembolization at an earlier point in the treatment may enable a greater proportion of patients to benefit from this therapy and provides the opportunity to combine this approach with suitable radiosensitizing chemotherapy regimens. Several, small, prospective trials of radioembolization in combination with chemotherapy have reported impressive results [22–28], and larger, phase II/III trials are ongoing [29].
In conclusion, the results reveal the significantly improved overall survival with the addition of radioembolization to BSC compared with BSC alone for patients who have failed multiple lines of systemic chemotherapy and for whom liver-dominant disease is the life-limiting condition.
References
Europe against Colorectal Cancer Declaration of Brussels 9 May 2007. http://www.future-health-2007.com/fileadmin/user_upload/Brussels_Declaration.pdf. Accessed July 2009
Lepage C, Remontet L, Launoy G et al (2008) French network of cancer registries (FRANCIM). Trends in incidence of digestive cancers in France. Eur J Cancer Prev 17:13–17
Manfredi S, Lepage C, Hatem C et al (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244:254–259
Golfinopoulos V, Salanti G, Pavlidis N, Ioannidis JP (2007) Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. Lancet Oncol 8:898–911
Folprecht G, Grothey A, Alberts S et al (2005) Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 16:1311–1319
Nordlinger B, Sorbye H, Glimelius B et al (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–1016
Grothey A, Sugrue MM, Purdie DM et al (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BriTE). J Clin Oncol 26:5326–5334
Tol J, Koopman M, Cats A et al (2009) Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 360:563–572
Gervais DA, Goldberg SN, Brown DB et al (2009) Society of interventional radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 20(7 Suppl):S342–S347
Popescu I, Alexandrescu S, Croitoru A, Boros M (2009) Strategies to convert to resectability the initially unresectable colorectal liver metastases. Hepatogastroenterology 56:739–744
Ricke J, Wust P, Wieners G et al (2005) Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavourable lesions for thermal ablation. J Vasc Interv Radiol 15:1279–1286
Denecke T, Lopez, Hänninen E (2008) Brachytherapy of liver metastases. Recent Results Cancer Res 177:95–104
Kennedy A, Coldwell D, Nutting C et al (2006) Resin 90Y microsphere brachytherapy for unresectable colorectal metastases: modern USA experience. Int J Radiat Oncol Biol Phys 65:412–425
Cosimelli M, Golfieri R, Cagol PP et al (2010) Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 103:324–331
Jakobs TF, Hoffmann RT, Dehm K et al (2008) Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol 19:1187–1195
Hoffmann RT, Jakobs TF, Kubisch C et al (2010) Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease–is it feasible? Eur J Radiol 74:199–205
Van den Eynde M, Flamen P, El Nakadi I et al (2008) Inducing resectability of chemotherapy refractory colorectal liver metastasis by radioembolization with yttrium-90 microspheres. Clin Nucl Med 33:697–699
Denecke T, Rühl R, Hildebrandt B et al (2008) Planning transarterial radioembolization of colorectal liver metastases with Yttrium 90 microspheres: evaluation of a sequential diagnostic approach using radiologic and nuclear medicine imaging techniques. Eur Radiol 18:892–902
Saltz L, Meropol N, Loehrer P et al (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208
Sangro B, Gil-Alzugaray B, Rodriguez J et al (2008) Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 112:1538–1546
Hendlisz A, Van den Eynde M, Peeters M et al (2010) Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 28:3687–3694
Dawood O, Mahadevan A, Goodman KA (2009) Stereotactic body radiation therapy for liver metastases. Eur J Cancer 45:2947–2959
Jonker DJ, O’Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
van Hazel G, Blackwell A, Anderson J et al (2004) Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 88:78–85
Sharma R, van Hazel G, Morgan B et al (2007) Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 25:1099–1106
van Hazel GA, Pavlakis N, Goldstein D et al (2009) Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using Yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol 27:4089–4095
Kuebler JP (2009) Radioembolization of liver metastases in patients with colorectal cancer: A nonsurgical treatment with combined modality potential. J Clin Oncol 27:4041–4042
Rose SC, Gulec SA (2009) Yttrium 90 radiomicrosphere therapy: ongoing clinical trials. J Interv Oncol 2:72–83
Acknowledgments
The authors thank the patients who participated in this study. We also thank Rae Hobbs for her editorial assistance on this manuscript, courtesy of Sirtex Medical Ltd. This trial was supported in part by Sirtex Medical Limited, Sydney, Australia. Ricarda Seidensticker and Max Seidensticker received travel fees. Jens Ricke and Maciej Pech received research grants and consultant fees from Sirtex Medical Limited, Sydney, Australia.
Conflict of interest
All other authors disclose any actual or potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seidensticker, R., Denecke, T., Kraus, P. et al. Matched-Pair Comparison of Radioembolization Plus Best Supportive Care Versus Best Supportive Care Alone for Chemotherapy Refractory Liver-Dominant Colorectal Metastases. Cardiovasc Intervent Radiol 35, 1066–1073 (2012). https://doi.org/10.1007/s00270-011-0234-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-011-0234-7