Abstract
Purpose
This study was designed to present our experience with percutaneous treatment of hepatic artery stenosis in orthotopic liver transplant patients and to evaluate the efficacy, technical outcomes, and mid-term clinical results of the procedure.
Methods
Twenty-two percutaneous transluminal angioplasties (PTAs) were performed in 19 liver transplant recipients at our institution between 1998 and 2010. Stents were placed into the hepatic/celiac artery in 16 PTAs, but balloon dilatation alone was performed in 6 because of the anatomical condition of the vessel. PTA/stenting was indicated in 17 patients because of elevated liver enzymes; 2 patients were asymptomatic. The objective of treating stenosis was prevention of long-term complications, including thrombosis.
Results
Technical success was achieved in all patients. There was only one complication: dissection of the treated artery without any subsequent adverse effects. In all patients, elevated liver enzyme levels improved after treatment. No restenosis was observed in any patient during a mean follow-up of 2.6 years (1 month to 5.5 years).
Conclusions
Percutaneous angioplasty/stent placement is a safe method for the treatment of hepatic artery stenosis after orthotopic liver transplantation, with a high technical success rate and promising mid-term results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Orthotopic liver transplantation offers the best survival option for many patients with acute liver failure, end-stage liver disease, liver tumors, and some metabolic diseases. Vascular and biliary complications are still important causes of graft failure despite improvements in operative technique, immunosuppression and organ preservation [1]. The most common of these complications include hepatic artery stenosis, thrombosis, or kinking [2, 3]. The case incidence of hepatic artery stenosis is reported to be 4–11% in adult transplant recipients; the incidence of hepatic artery thrombosis varies from 3 to 20% [2, 4]. Stenosis or thrombosis of the portal vein or stenosis of the inferior vena cava occurs in 1–2% of patients. A higher incidence of vascular complications is reported in pediatric recipients and retransplanted patients [2]. Hepatic artery stenosis can lead to thrombosis, a serious complication that compromises long-term graft function and survival if it is not treated. Arterial occlusion may lead to bile duct ischemia because the supply of biliary tree is exclusively from the hepatic arterial circulation, whereas gastroduodenal artery is interrupted after transplantation [5, 6]. Vascular complications increase the morbidity and mortality of transplanted patients [2]. The traditional treatment options of symptomatic hepatic artery stenosis include anticoagulation, surgical revascularization, or retransplantation. However, use of percutaneous interventional procedures has increased during the past decade, following improvements in endovascular techniques.
Patients and Methods
Orthotopic liver transplantation was performed in 800 patients with end-stage liver disease between 1996 and 2010. Hepatic/celiac artery stenosis occurred in 19 patients (2.4%) after transplantation. Arterial stenoses were diagnosed in three patients with a retransplant liver graft. These patients all underwent percutaneous treatment, with 22 arterial stenoses treated by PTA with stent implantation or by angioplasty alone. Thirteen patients were male and six were female. The mean age was 42 (range, 3–62) years, and all were adults except one 3-year-old child. The end-stage liver disease resulting in liver transplantation was cirrhosis caused by hepatitis B or C, or alcoholic cirrhosis in six patients, autoimmune cirrhosis in one patient, and cirrhosis associated with Wilson disease in two patients. One pediatric patient had biliary atresia. Other reasons for transplantation were primary sclerosing cholangitis, Budd–Chiari syndrome, congenital liver fibrosis, primary hepatocellular carcinoma in cirrhosis, and hemangioendothelioma. Three patients underwent transplantation because of acute liver failure (Table 1).
Hepatic artery stenosis was diagnosed by routine Doppler ultrasonography (high velocity flow in the stenosis or low resistance level index) and/or by CT angiography (narrowing of >50% of vessel diameter). All patients underwent CT angiography before PTA to evaluate the vascular anatomy after transplantation and confirm the location of the stenosis. The average time between transplantation and endovascular treatment was 2.7 months (range, 3 days to 6 months). Except for two patients who were asymptomatic, PTA of the hepatic artery was prompted by deterioration of liver function. The median values of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 1.5 μkat/l (range, 0.51–147) and 2.67 μkat/l (range, 0.69–80), respectively. In the two patients who were without symptoms, tight stenosis of the hepatic artery was detected by Doppler ultrasonography examination during routine posttransplantation follow-up. PTA was performed to prevent acute thrombosis or long-term complications. Seventeen PTA procedures were performed by a transfemoral approach, four cases via the left brachial artery, and one hepatic artery stent was placed using surgical cut-down of the vessel due to extreme arterial tortuosity. Fourteen treated stenoses were located in the anastomosis of the donor and the recipient hepatic artery, five were located distally in the donor vessel, and three were in the celiac artery (Table 1). In nine of the patients, it was necessary to treat stenosis of the biliary duct or a biliary leak simultaneously by an endoscopic or percutaneous approach.
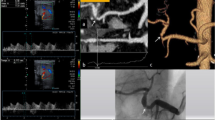
Stent placement was successful in all 16 procedures. Eleven self-expandable stents (Smart stents, Cordis, USA or Wallstents, Boston Scientific, USA) were placed into hepatic or celiac artery stenoses (Figs. 1, 2). In one patient, Smart stents were placed into stenoses in both the celiac and hepatic arteries. In five patients, balloon expandable stents were used: two short Express stents (Boston Scientific, USA) were placed into celiac artery stenoses; a Palmaz Genesis stent (Cordis, USA) was placed into a short stenosis in a hepatic artery anastomosis; an AVE coronary stent (Medtronic, USA) was used to treat a stenosis in the hepatic artery in the 3-year-old child. Another coronary stent (Pro-Kinetic Energy, Biotronic, Swiss) was placed in the hepatic artery anastomosis of an adult patient. In six cases, PTA was done alone without stent placement because of significant tortuosity of the feeding arteries (Fig. 3). All of these stenoses were very difficult to cross by guidewire and balloon catheter alone. In one patient, a Smart stent was placed into the anastomotic stenosis of hepatic artery and 5 months later a stenosis of the right hepatic artery was dilated by balloon only. PTA of an anastomotic stenosis and a donor artery stenosis without stent placement was performed in one patient due to tortuosity of vessels (Table 1). All patients received 5,000 units of heparin during the procedure. Additional 5,000-unit doses were given subcutaneously in the evening after the angioplasty and the next morning. All patients took 100 mg of acetylsalicylic acid orally daily for at least 3 months after the procedure.
Male patient 30 days after liver retransplantation complicated by a biliary leak. Smart stent was placed into the hepatic artery stenosis with a good technical result. The biliary leak was treated by percutaneous biliary drainage. The patient has good liver function 4 years after the procedure; the leak healed completely
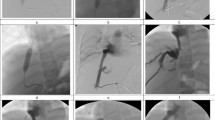
Male patient 24 days after liver transplantation with a tight anastomotic stenosis of the hepatic artery and no clinical symptoms. Due to tortuosity of hepatic artery, only a balloon dilatation was performed with a good result. The patient has been followed 17 months after PTA without evidence of restenosis
All patients were followed-up in the outpatient department of our hospital. The examination protocol for liver transplant patients includes physical and laboratory examinations and Doppler ultrasound monthly for the first year after transplantation and then every 3 months afterward. Liver biopsies were performed at 1, 2, 3, 5, 7, and 10 years after transplantation. Follow-up was modified according to clinical status of the patient.
Results
Technically successful dilatation (defined as a resultant vessel diameter of at least 70% of the nonstenotic segment) was achieved in all 22 lesions (Table 1). Only two residual stenoses of 20% of artery diameter remained in two patients dilated without stent placement. In one patient (5.3%), dissection of the hepatic artery occurred during PTA without affecting the clinical outcome. This patient is currently on antiplatelet therapy, has normal blood flow in the hepatic artery on Doppler ultrasonography, and good liver function 4.5 years after PTA. A large hematoma that developed in this patient in the puncture site was treated by prolonged compression only. No other complications occurred in our group of patients.
No restenosis of any dilated artery was diagnosed during a mean follow-up of 2.6 years (range, 1 month to 5.5 years). Patients were followed by Doppler ultrasound; serum liver enzymes were obtained routinely. Both ALT and AST values improved in all patients within a month after the procedure. The median values of liver enzymes 1 month after percutaneous therapy were: AST 0.48 (range, 0.22–2.66) μkat/l, ALT 0.66 (range, 0.25–4.12) μkat/l (Figs. 4, 5). These changes were statistically significant (Wilcoxon pair test, p < 0.01). Eleven transplanted patients from our group are alive with good liver function. The only treated child was lost to follow-up. Six patients died from causes unrelated to the hepatic artery intervention: one from carcinoma of the neck, three because of acute, corticoid-resistant cellular rejection (but with patent treated arteries), and two patients from severe sepsis. Four of these patients died within 3 months after transplantation, three had been transplanted because of acute liver failure, and in one patient there had been a second retransplantation in a very short time period. Another female patient was retransplanted after recurrence of primary disease (cirrhosis caused by hepatitis C) 2 years after PTA (Table 1). If we exclude the four patients with acute cellular rejection who died within 3 months after transplantation, the mean follow-up of the remaining 15 patients would be 3.2 years without detected restenosis of hepatic artery.
Discussion
Hepatic artery stenosis has been reported in 31% of the arterial complications of orthotopic liver transplantation, less frequently than hepatic artery thrombosis (58%) and more frequently than kinking 6%) [3]. Most hepatic artery stenoses are located close to the surgical anastomosis [3]. Proximal stenoses are in the recipient or native vessels from the celiac axis to anastomosis, distal stenoses occur in donor vessels [3]. The cause of hepatic artery stenosis can be surgical technique, vascular clamp injury; microvascular lesions associated with preservation solutions, allograft rejection, or it can remain unexplained [2]. Clinical presentation of stenosis includes deterioration of graft function with elevation of liver enzymes, but approximately 20–27% of stenoses can be asymptomatic [2, 7], so routine Doppler ultrasound is necessary to detect them. Two patients with tight stenosis detected shortly after transplantation were symptom-free and the PTA/stent procedure was performed to prevent potential hepatic artery thrombosis.
We used Doppler ultrasonography as a routine noninvasive screening method, although its sensitivity is estimated at only 85% [2]. CT or MR angiography also can visualize stenosis and also anatomical conditions of transplant graft and vascular anastomosis. Liver biopsy also can depict ischemia. Concomitant ischemic biliary complications (biliary stenosis, leak) are quite frequent, and significantly more prevalent in patients with hepatic artery stenosis with 67% abnormal cholangiographic findings [8]. Nine of our 18 adult patients (50%) required percutaneous or endoscopic biliary tract drainage.
Percutaneous treatment (PTA/stent) of hepatic artery stenosis can achieve lower morbidity compared with surgery [2]. The technical success rate of hepatic artery angioplasty is reported to be 80–90% [3]. Most technical failures are due to inability to cross the lesion with the balloon or stent secondary to a tortuous or kinking artery or associated arterial spasm. The five patients with stenoses in tortuous arteries were all successfully dilated by balloon without stent placement. In three of them, a brachial artery approach was helpful to reach the stenosis. The other cause of technical failure is arterial recoil [3]; however, in our patients no recoil occurred. Multiple stenoses are not considered a contraindication for percutaneous treatment [9]. One female patient in our study had a significant stenosis in the right hepatic artery and another in the left branch of the hepatic artery; both were dilated by balloon only, with good results. Some authors have reported better results with stent placement than with angioplasty alone [2], but in-stent restenoses are quite frequent, reported in approximately 25% of cases [2, 7]. No instances of restenosis with either technique were detected in our series during follow-up. Thus, it may be preferable to perform angioplasty alone without stent placement in patients with extremely tortuous vessels.
Most vascular complications develop within 3 months after transplantation [10]. Some reports recommend percutaneous intervention not to be performed earlier than 3 weeks after transplantation because of risk of rupturing vessel anastomoses [7]. According to other authors, PTA can be performed as soon as 1 week after transplantation [11]. We placed a stent in one of our patients 3 days after transplantation, but the stenosis was located in the celiac trunk far from the vascular anastomosis. Celiac artery stenosis/compression that can impair the hepatic blood flow is found in approximately 10% of liver transplant recipients [12]. It may exacerbate graft ischemia because of absence of collateral circulation in transplanted patients. The risk of thrombosis in nontreated hepatic artery stenosis is reported to be 65% within 6 months but decreases to 19% with successful treatment [3]. It is for this reason that PTA of asymptomatic stenosis is recommended.
Arterial stenosis is strongly associated with increasing levels of liver enzymes. Significant decreases of AST and ALT after successful PTA occurred in all of our patients (except for two patients who were asymptomatic before PTA/stenting). The improvement of liver function (expressed by ALT and AST values) was not caused by PTA only, because other medical liver-protective therapies were employed as well; but as the values of both ALT and AST enzymes decreased in all patients after revascularization, its effect seems to be unquestionable.
The periprocedural complication rate is reported to be 5–30% [3, 13] depending on vessel tortuosity and stenosis location and corresponds well with our results: one complication in 19 patients (5.3%). A higher complication rate is reported in tortuous lesions, tandem, and distal stenoses [3]. The most frequent complications include hepatic artery dissection, thrombosis, and pseudoaneurysm. Serious complications are rupture or perforation of hepatic artery [3]. In our patients, only one complication (dissection of the hepatic artery) occurred and was without subsequent blood flow alteration.
Conclusions
Percutaneous interventional therapy is a safe treatment option for hepatic artery stenosis in liver transplant patients. In our patients, it had an excellent technical success rate with promising mid-term clinical results. PTA/stent can be the first method in hepatic artery stenosis treatment, with surgical correction or retransplantation used in the event of failure or complications of PTA. In tortuous vessels PTA alone without stent implantation can be employed as an alternative method.
References
Cheng YF, Chen CL, Chen YS et al (2000) Interventional radiology in the treatment of post-liver transplant complications. Transplant Proc 32:2196–2197
Da Silva RF, Raphe R, Felício HC et al (2008) Prevalence, treatment, and outcomes of the hepatic artery stenosis after liver transplantation. Transplant Proc 40:805–807
Saad WEA (2007) Management of hepatic artery steno-occlusive complications after liver transplantation. Tech Vasc Interv Radiol 10:207–220
Boyvat F, Aytekin C, Harman A et al (2008) Endovascular stent placement in patients with hepatic artery stenoses or thromboses after liver transplant. Transplant Proc 40:22–26
Bekker J, Ploem S, De Jong KP (2009) Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant 9:746–757
Hoffer FA, Littlewood Teele R, Lillehei CW et al (1998) Infected bilomas and hepatic artery thrombosis in infant recipients of liver transplants. Radiology 169:435–438
Ueno T, Jones G, Martin A et al (2006) Clinical outcomes from hepatic artery stenting in liver transplantation. Liver Transpl 12:422–427
Orons PD, Sheng R, Zajko AB (1995) Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. AJR Am J Roentgenol 165:1145–1149
Yang Y, Hua L, Bin-sheng FU et al (2008) Hepatic artery complications after orthotopic liver transplantation: interventional treatment or retransplantation? Chin Med J 121(20):1997–2000
Miraglia R, Maruzzelli L, Caruso S et al (2009) Interventional radiology procedures in adult patients who underwent liver transplantation. World J Gastroenterol 15:684–693
Kodama Y, Sakuhara Y, Abo D et al (2006) Percutaneous transluminal angioplasty for hepatic artery stenosis after living donor liver transplantation. Liver Transpl 12:465–469
Jiang Z, Liang T, Feng X et al (2008) Arcuate ligament syndrome including hepatic artery thrombosis after liver transplantation. Hepatobiliary Pancreat Dis Int 7(4):433–436
Chen GH, Wang GY, Yang Y et al (2009) Single-centre experience of therapeutic management of hepatic artery stenosis after orthotopic liver transplantation. Report of 20 cases. Eur Surg Res 42(1):21–27
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00270-011-0183-1
Rights and permissions
About this article
Cite this article
Jarmila, L., Jan, P. Percutaneous Transluminal Angioplasty of Hepatic Artery Stenosis in Patients After Orthotopic Liver Transplantation: Mid-term Results. Cardiovasc Intervent Radiol 34, 1165–1171 (2011). https://doi.org/10.1007/s00270-010-0082-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-0082-x