Abstract
The purpose of this study was to evaluate the effectiveness of colorectal cancer (CRC) liver metastasis radioembolization with yttrium-90 (Y90), assessing toxicity and survival rates in patients with no response to chemotherapy through our 3-year experience. From February 2005 to January 2008, we treated 41 patients affected by CRC from a cohort of selective internal radiation therapy patients treated at our institution. All patients examined showed disease progression and arrived for our observation with an abdominal CT, a body PET, and a hepatic angiography followed by gastroduodenal artery coiling previously performed by us. We excluded patients with a bilirubin level >1.8 mg/dl and pulmonary shunt >20% but not patients with minor extrahepatic metastases. On treatment day, under fluoroscopic guidance, we implanted a dose of Y90 microspheres calculated on the basis of liver tumoral involvement and the body surface area formula. All patients were discharged the day after treatment. We obtained, according to Response Evaluation Criteria on Solid Tumors, a complete response in 2 patients, a partial response in 17 patients, stable disease in 14 patients, and progressive disease in 8 patients. In all cases, we obtained a carcinoembryonic antigen level decrease, especially in the week 8 evaluation. Technical success rate was 98% and technical effectiveness estimated at 3 months after treatment was 80.5%. Side effects graded by Common Terminology Criteria on Adverse Events were represented by one grade 4 hepatic failure, two grade 2 gastritis, and one grade 2 cholecystitis. The median survival and the progression-free survival calculated by Kaplan–Meier analysis were 354 and 279 days, respectively. In conclusion, according to our 3-year experience, Y90 SIR-Spheres radioembolization is a feasible and safe method to treat CRC liver metastases, with an acceptable level of complications and a good response rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the most common gastrointestinal tumor and, with almost 437,000 annual deaths worldwide, represents the third most common malignancy in the developed world [1]. In 60% of cases patients suffering from CRC will develop liver metastases, and in 20% of these, the liver represents the only site of the disease at death [2]. Surgical resection is the only curative possibility to obtain long-term survival. It is generally admitted that 10% to 15% of patients with synchronous colorectal liver metastasis will benefit from hepatic resection [3].
Unfortunately, only selected patients may undergo surgery and therefore new palliative treatment options, besides systemic chemotherapy, have been developed in recent years. Cryotherapy and radiofrequency ablation have shown promising results but they are feasible only for patients with a limited number of lesions [4]. Hepatocytes receive blood supply from the hepatic artery and primarily from the portal vein but liver tumors and metastases are predominantly supplied from the hepatic artery (90%) [5]. On this basis, selective internal radiation therapy (SIRT) represents a new treatment option based on the injection of microspheres into the hepatic artery. These resin microspheres contain yttrium, a high β-emitting radioisotope, and are injected with the aim of inducing microembolization of the hepatic arterioles and local irradiation.
According to animal studies and also to studies regarding dose estimation from explanted liver, the average dose administered to liver tumors is 200–300 Gy [6, 7], while standard percutaneous radiation therapy of the liver achieves only 30–35 Gy, which is not sufficient to produce a relevant antitumor effect [8].
The aim of our study was to evaluate the feasibility and to analyze the results of SIRT in patients with liver metastases from CRC nonresponsive to standard chemotherapy therapeutic protocols.
Materials and Methods
Prior to undertaking our procedures and to collecting all data retrospectively, Institutional Review Board approval was obtained.
Patient Demographics
From February 2005 to January 2008, at the Interventional Radiology Unit we treated 105 patients affected by liver tumors. Among these 41 were caused by CRC. Patients were 30 males and 11 females, with a mean age of 61.2. In all cases they underwent several unsuccessful chemotherapy cycles. There were no indications for other treatments such as radiofrequency ablation, considering the size of the lesions and their distribution in the liver.
All patients presented asymptomatic at the time of treatment and their median ECOG (Eastern Cooperative Oncology Group) status was 0.7. Before any kind of treatment, all patients received all information regarding the procedure and risks connected with the treatment, including side effects, and signed a consent form allowing us to proceed.
In all patients we detected multiple lesions, with bilobar involvement in 39 cases. Other metastatic involvement apart from the liver was present in four patients (9.7%) and included pathologic lymph nodes and bone metastases.
The aim of selection was thus based on acceptance of patients who had already received and failed standard first-line FOLFOX (folinic acid, fluorouracil [5-FU], xaliplatin), second-line FOLFIRI (folinic acid, 5-FU, irinotecan), and third-line chemotherapies. All patients were evaluated by a clinical oncologist, a radiation oncologist, and an interventional radiologist before the beginning of treatment.
Inclusion and Exclusion Criteria
Eligible patients had a confirmed diagnosis of colon or rectal adenocarcinoma with liver-involving disease, were able to give informed consent, had an adequate bone marrow reserve (granulocytes, >1500/μl; platelets, >60,000/μl), adequate hepatic function (total bilirubin, <1.8 mg/dl; ALT/AST or alkaline phosphatise, less than five times the upper limit of normal), satisfactory pulmonary function (FEV1, >1 L), and no contraindication for selective angiography.
A CT scan and a FDG-PET were performed to assess liver disease and to evaluate extrahepatic metastatic disease. Levels of carcinoembryonic antigen (CEA), a disease-specific tumoral marker, were obtained in all patients. Patients with lung shunting >20% and a bilirubin level >1.8 mg/dl were excluded from treatment.
Study Protocol
Selected patients underwent visceral angiography by brachial access using a 4-Fr sheath (Terumo) in order to be discharged a few hours after the procedure. The study was performed to evaluate the vascular anatomy of the liver. During selective catheterization, branches of the hepatic artery to the GI tract, such as the gastroduodenal artery and the right gastric artery, were coil embolized using 2 × 10- and 2 × 20-mm microcoils (Target vascular; Boston Scientific) to prevent yttrium-90 (90Y) reflux to the stomach and duodenum.
At the end of the procedure a dose of 150 MBq technetium macroaggregated (Tc-99 m) albumin (MAA) was administered through a selective catheter to perform single-photon-emission computed tomography (SPECT) at the Nuclear Medicine Department, to detect arteriovenous shunts from the hepatic arterial system to the pulmonary system or ectopic implantation into the GI tract. This test serves as a surrogate since MAA particles provide approximately the same size as (Y90) microspheres, predicting possible undesired implantation of the microspheres outside the target vessel territory.
Dosimetry
Each manufacturer recommends a different way to calculate the appropriate 90Y microspheres to administer. A dose of 2 GBq contains approximately 50 million microspheres and is considered to be the standard dose for liver involvement <25%. The dose is usually 2.5 Gbq for disease spread up to 50% and 3 GBq for liver replacement >50%.
The CT scan also allowed us to calculate the tumor involvement of the liver, which indicated the required activity for treatment and provided identification of tumor distribution. Patients who underwent the sequential procedure were then treated selectively from the left or from the right hepatic artery. For whole-liver treatment administration was from the common hepatic artery.
The typical activity at the time of infusion is 50 Bq/resin sphere. On treatment day, proceeding with no sedation, the dose of SIR-Spheres was hand-injected over a 20- to 30-min period in a very low-flow injection by selective hepatic catheterization performed using a Progreat microcatheter (Terumo).
Statistical Method
According to the Kaplan–Meier analysis we evaluated the median survival and the progression-free survival rate for our cohort of patients and analyzed technical success and technique effectiveness. Furthermore, we stated our complications and side effects according to the Common Terminology Criteria for Adverse Events (CTAE).
Tumor Response
Tumor response was assessed both by changes in CEA levels and by CT scanning modification, defined according to Response Evaluation Criteria in Solid Tumors (RECIST), as follows.
-
Complete response (CR): disappearance.
-
Partial response (PR): 30% decrease.
-
Stable disease (SD): neither PR nor PD criteria met.
-
Progressive disease (PD): 20% increase; no CR, PR, or SD documented before increased disease.
Follow-up was scheduled in 8- and 12-week CT and PET controls. The interval between lobar treatments was 4 to 6 weeks. After the first 8 weeks we evaluated the possibility of re-treatment.
Criteria for Re-Treatment
We needed to re-treat 31 patients. The criteria we used for re-treatment was based on the imaging response of treatment after 8 weeks. In the case of PR and SD we always attempted re-treatment according to the oncologist, to improve the response. In the case of PD a salvage attempt was performed, increasing the dose slightly.
Results
On treatment day, all patients underwent SIRT, receiving a lobar or a whole-liver infusion. All patients were treated after all surgical options were ruled out and every medical treatment failed. In all cases, antiangiogenic agents were suspended with the intent to increase the tumoral 90Y uptake.
Radiation
The mean delivered activity registered was 1.82 GBq (Table 1). As reported in Table 1 two patients received selective lobar treatment, 32 whole-liver treatment, and 7 sequential treatment of the right lobe followed by the left lobe, performed after 30 days to reduce the risk of acute liver toxicity. Whole-liver treatment was attempted in our early experience in patients affected by bilobar metastases, while recently we have preferred to perform sequential infusion to decrease the risk of complications.
Liver Involvement
Estimated liver involvement, calculated based on CT scans and PET, was <25% in 25 patients, between 25% and 50% in 6 patients, and >50% in 10. Four patients were affected by extrahepatic metastatic disease, in particular, lymph nodes, but it was generally minor and not life-threatening.
Tumor Response
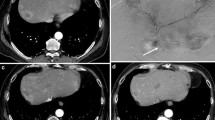
All patients were studied with an 8-week follow-up by means of a CT scan and PET (Figs. 1 and 2). According to our response rates, we achieved a CR in 2 patients (4.8%), a PR in 17 patients (41.5%), SD in 14 patients (36.2%), and PD in 8 patients (19.5%). In all cases we observed a decrease in CEA levels at follow-up 8 weeks after SIRT. The median CEA level among our patient cohort was 4.2 μg/L) before treatment and 2.1 μg/L after treatment. In particular, in 25 patients we registered a value <2.5 μg/L, and in 16 patients <2.0 μg/L. In patients who underwent sequential treatment, we observed reduced contrast medium uptake in the right lobe upon selective hepatic angiogram, performed to treat the left lobe. In this phase, we observed the first treatment-related contrast medium uptake modifications.
The median survival calculated by Kaplan–Meier analysis was 354 days (Fig. 3) and the progression-free survival was 279 days (Fig. 4). We did not register any 30-day mortality among our cohort of patients. Ten patients are still alive. The others died from disease progression. In five patients we detected the occurrence of extrahepatic disease defined by pulmonary (three patients) and brain (two patients) metastases.
Our technical success rate was 98%. In one patient we had to stop the infusion as the patient complained of pain during Y90 injection. The technical effectiveness estimated at 3 months after treatment was 80.5%; nine patients did not seem to benefit from the treatment performed.
Complications
All side effects are expressed according to the CTCAE.
Immediate Complications
We registered mild abdominal pain or nausea in five patients 12 h after the procedure. They were treated with prompt pain relief or antiemetic medications.
Early Complications
We observed one grade 2 cholecystitis after 25 days, which was managed with medical therapy.
Late Complications
Two patients complained of grade 2 gastritis, 4 and 6 weeks after treatment, respectively, and were also treated with medical therapy. In one patient treated in our early experience, we observed grade 4 hepatic failure 40 days after treatment, probably due to radiation-inducted hepatitis.
Summary
We observed one major hepatic complication related to hepatic failure and three minor extrahepatic complications due to two cases of grade 2 gastritis and one of grade 2 cholecystitis.
It is significant to report that no complications occurred in patients treated with the sequential procedure, although their number was low, and no complications were registered after the brachial approach during pretreatment evaluation.
Discussion
Autopsy data demonstrated that patients suffering from CRC die from their liver disease, and their clinical course demonstrates that progression of liver metastases is more rapid than that of most other sites, strongly influencing the prognosis [2]. Approximately 60% of diagnosed patients present with the liver as the predominant site of metastatic spread [21].
The first 90Y treatment in patients with liver metastases from CRC was published in 1964 by Ariel [9]. Combined SIRT and chemotherapy provided better response rates than either treatment alone. In the subsequent decades, several authors have reported their experiences with the use of radioactive isotopes for treatment of liver cancer [10–20]. In the beginning of microsphere manufacture, leakage of radiation was a problem, which led oncologists to abandon this treatment option. The development of TheraSpheres (glass) and SIR-Spheres (resin) in the late 1980s rekindled the interest in this treatment option. MAA pretreatment evaluation produced encouraging results, preventing eventual complications and increasing the use of both resin and glass spheres [22].
Complications are usually due to radiation-associated injury, a major concern related to external-beam radiotherapy. The incidence of potentially lethal radiation hepatitis is approximately 75% with doses exceeding 40 Gy to the whole liver, using conventional external-beam radiation [23]. The tolerance of normal liver parenchyma to radiation represents the key issue. The maximum acceptable dose to the whole liver, 35 Gy, is much lower than the 70 Gy required to destroy adenocarcinoma metastases [22]. Therefore the tumoricidal dose is difficult to achieve using external-beam radiation therapy. Stereotactic radiation may represent a possible alternative approach, but usually it proves unfeasible because of a multifocal and irregular distribution of liver metastatic disease. SIRT provides much higher radiation doses at the tumor level [24], with a relatively wide safety margin clearly demonstrated in numerous studies [25]. It has been demonstrated that the preferred microsphere location is the growing rim of the tumor, since its central portion is usually necrotic and therefore does not have the good blood supply essential for 90Y treatment [7].
The radiation damage to the liver associated with SIRT is microscopically due to microinfarcts and a chronic inflammatory infiltrate dominating at the portal triads, minimizing the damage to the central vein observed in radiation hepatitis from external-beam radiation [26]. Radiation hepatitis represents one of the most serious complications. In one of their studies, Stubbs et al. [4] suggested delivery of the activity sequentially in a single treatment session. Other important complications are represented by gastric or duodenal ulcers, resulting from Y90 reflux into the GI vascular bed, and radiation pneumonitis, estimated as a lung-absorbed dose of 30 Gy, associated with severe shunting [27], preventable through 99mTc MAA pretreatment evaluation.
According to our results, in a significant number of patients (46.2%) we obtained a CR/PR based on RECIST. These data differ from those reported in three recent studies, by Kennedy et al. [22], Jakobs et al. [28], and Cosimelli et al. [29] Kennedy et al. reported a PR rate of 35%; Jakobs et al. and Cosimelli et al., a PR rate of 17% and 12,5% respectively. These differences are not apparently correlated with inclusion criterion variations or with treatment modifications. The reason seems to be related to the extent of metastatic liver involvement of the patients.
Excluding the first treatments, performed mainly in a salvage setting, patients arrived for our observation with a hepatic spread usually <25%. Jakobs et al. described a significantly improved survival rate in patients with <25% tumor involvement of the liver. It can be assumed that those patients with small tumor involvement of the liver might more likely present SD or PR at first follow-up after SIRT. However, the median survival of our patients (354 days) did not differ substantially from the data published by Jakobs et al. and Kennedy et al. (315 days). This might appear to be contradictory but can be explained by the heterogeneity of our cohort, composed of patients treated during our early experience, and suffering from clinically advanced disease and tumor involvement of the liver frequently >50%, and patients treated more recently, with a reduced tumor load of the liver (<25%).
This may also explain our SD rate, 36%, considerably lower than those reported by Jakobs et al. (61%), Kennedy et al. (55%), and Cosimelli et al. (75%). Considering these data it would be reasonable to conduct a prospective study based on tumor involvement of the liver >25% as a treatment exclusion criterion. In a study on unresectable chemorefractory liver metastases from different types of primary tumors, Sato et al. reported CR/PR, SD, and PD rates of 42.8%, 47%%, and 10.2%, respectively. The high PR rate is significantly related to the rate of patients with tumor involvement of the liver <25% (80%). These data, apparently similar to our series, although referred to different types of primary tumors, were achieved based on WHO criteria. However, good agreement described between WHO criteria and RECIST was reported [30], making the data published by Sato et al. comparable to our results. A reduction in size assessed by cross-sectional imaging does not always reflect the true response of tumors to treatment. This happens because various peri- and intratumoral changes occur after Y90 radioembolization, such as hemorrhage, peritumoral edema, and ring enhancement. Therefore tumor response might be preferentially detected by volumetric assessment or metabolic assessment. This might mean that patients rated as having SD using cross-sectional imaging would be assessed as having a PR based on metabolic information. To improve the assessment of tumor response it is recommended that FDG-PET or functional MRI (with diffusion-weighted sequences) be an integral part of the posttreatment evaluation [31–34]. Considering this fundamental aspect we ask all patients to undergo PET together with CT scan 8 weeks after treatment in order to have a complete evaluation of our response, and even if the response evaluation was made based on the CT scan by RECIST, PET data helps to define better the treatment response.
Sato et al. additionally emphasized that the ECOG score represents an important prognostic factor. In their experience patients with an ECOG score of 0 showed a median survival of 731 days, vs. 137 days for patients with an ECOG score >0. This trend seems to be independent of tumor type and appears to be so highly significant that an ECOG score >0 may represent a prognostic factor for performing liver-targeted therapies. Our experience widely confirms this consideration. Patients treated early in our experience with an ECOG score >0 and liver metastatic spread >25% showed survival rates significantly lower than those of patients treated more recently, although it was not possible to obtain these parameters for all patients.
Another fundamental criterion providing relevant prognostic information is represented by tumoral marker decrease.
According to Stubbs et al. a CEA reduction of >30% from the initial value is strongly connected to an improved survival rate, especially if radioembolization is combined with hepatic arterial chemotherapy [4]. Jackobs et al. reported a median survival rate in patients with CEA reductions, although affected by extrahepatic spread of the disease, of 19.1 months, vs. a median survival rate of 12.3 months for all patients treated [28].
Probably the most important matter in the future role of SIRT regards the combination of 90Y treatment with chemotherapy. According to our experience and early reports, this procedure was initially performed in a salvage setting, once every other therapy failed and no further options were available. Gray et al. reported a randomized study of 74 patients affected by CRC liver metastases treated with SIRT and infusion of floxiuridine (FUDR) vs. FUDR alone [35]. Patients who received the combined therapy had a median time of disease progression significantly longer compared to that of patients who received hepatic arterial chemotherapy (HAC) alone: the 1-, 2-, 3-m and 5-year survival rates in patients treated with SIRT were 72%, 39%, 17% and 3.5%, respectively, compared to 68%, 29%, 6.5%, and 0% for HAC alone.
Other studies were developed with the same aim of assessing the usefulness of combined SIRT and chemotherapy. The Eastern Cooperative Oncology Group organized a phase II trial to study the combination of SIRT and 5-FU/leucovorin. This represents the necessary step in considering the potential benefits of the newest chemotherapeutic agents combined with the established effectiveness of resin microspheres in the treatment of liver metastatic disease, assessed by RECIST and clinical evaluation as reported by different authors [36]. At the 2005 ASCO GI Cancer Symposium, Van Hazel et al. proposed results of phase I studies on concurrent treatment based on oxaliplatin, 5-FU, leucovorin, and SIRT during the first week of chemotherapy. Response on CT was significant for 10 of 11 patients [37]. In a randomized phase II study of 21 patients, radioembolization administered as first-line treatment along with systemic chemotherapy significantly increased the treatment-related response (10 vs. 0 patients showed a PR on follow-up CT), time to disease progression (18.6 vs. 3.6 months) and survival (29.4 vs. 12.8 months) compared with chemotherapy alone [38].
The authors also focused their studies on agents such as bevacizumab and cetuximab antiangiogenics. These growth factor inhibitors may control tumor size but their effect on the vascular system may affect microsphere uptake. On this basis, we ask our patients to suspend antiangiogenic drugs at least 4 weeks before treatment day.
Planning also represents an important aspect of SIRT. An appropriate vascular and anatomic evaluation must be achieved before deciding on any selective therapy. Vascular anatomy can be extremely variable and the knowledge of each patient’s liver perfusion is absolutely mandatory, as recently reported [39].
In the future, perspective dose planning will allow advancement in the treatment protocol, with the aim of administering a more precise dose at the tumor level.
New embolic particles, such as HepaSphere, a new spherical embolic material developed in a dry state that absorbs fluids and adapts to the vessel wall, leaving no space between the particle and the arterial wall, are now being tested in animal models [40].
In conclusion, on the basis of our experience and the numerous articles published to date, SIRT has proven its effectiveness and future prospects appear to be encouraging. Phase II trials are actually examining the effectiveness of combined SIRT/chemotherapy treatment so that in the future Y90 radioembolization will not represent a salvage therapeutic option but will contribute to improving patient survival rates, delaying disease progression. Liver disease downstaging will be the ideal aim of the treatment, to allow more patients to undergo surgery. According to the current trends, the dose will be checked precisely and the procedure will be performed as selectively as possible, with the aim of treating the tumor aggressively and protecting the healthy tissue surrounding it.
References
Pisani P, Parkin DM, Bray F et al (1999) Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 83:18–29
Weiss L, Grundmann E, Torhorst J et al (1986) Haematogenus metastasis patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol 150:195–203
Schlag PM, Benhidjeb T, Stroszczynski C (2002) Resection and local therapy for liver metastases. Best Pract Res Clin Gastroenterol 16:299–317
Stubbs RS, O’Brien I, Correia MM (2006) Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. Aust NZ J Surg 76(8):696–703
Breedis C, Young G (1954) The blood supply of neoplasms in the liver. Am J Pathol 30(5):969–977
Ingold JA, Reed GB, Kaplan HS et al (1965) Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med 03:200–208
Kennedy AS, Nutting C, Coldwell D et al (2004) Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 60(5):1552–1563
Lawrence TS, Robertson JM, Anscher MS et al (1995) Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 31:1237–1248
Ariel IM (1964) Radioactive isotopes for adjuvant cancer therapy. Arch Surg 89:244–249
Grady ED, Sale WT, Rollins LC (1963) Localization of radioactivity by intravascular injection of large radioactive particles. Ann Surg 157:97–114
Nolan T, Grady ED, Crumbley AJ (1973) Regional internal radiation for hepatic cancer. Minerva Oncol 1:104–106
Nolan T, Grady ED, Crumbley AJ et al (1975) Internal hepatic radiotherapy: I. Organ distribution of colloidal Cr32 PO4 injected into a peripheral vein, the portal vein, or the arterial supply of the gastrointestinal tract in the rat. Am J Roentgenol Radium Ther Nucl Med 124:590–595
Grady ED, Nolan T, Larose JH et al (1975) Internal hepatic radiotherapy: II. Intra-arterial radiocolloid therapy for hepatic tumors. Am J Roentgenol Ther Nucl Med 124:596–599
Grady ED (1979) Internal radiation therapy of hepatic cancer. Dis Colon Rectum 22:371–375
Ariel IM (1965) Treatment of inoperable primary pancreatic and liver cancer by the intraarterial administration of radioactive isotopes (Y90 radiating microspheres). Ann Surg 162:267–278
Ariel IM, Pack GT (1967) Treatment of inoperable cancer of the liver by intra-arterial radioactive isotopes and chemotherapy. Cancer 20:793–804
Mantravadi RV, Spigos DG, Tan WS et al (1982) Intraarterial yttrium 90 in the treatment of hepatic malignancy. Radiology 142:783–786
Ariel IM, Padula G (1982) Treatment of asymptomatic metastatic cancer to the liver from primary colon and rectal cancer by the intraarterial administration of chemotherapy and radioactive isotopes. J Surg Oncol 20:151–156
Blanchard RJW (1983) Treatment of liver tumours with yttrium-90 microspheres. Can J Surg 26:442–443
Gyves JW, Ziessman HA, Ensminger WD et al (1984) Definition of hepatic tumor microcirculation by single photon emission computerized tomography (SPECT). J Nucl Med 25:972–977
Sasson AR, Sigurdson ER (2002) Surgical treatment of liver metastases. Semin Oncol 29(2):107–118
Kennedy AS, Coldwell D, Nutting C et al (2006) Resin Y90 microspheres brachytherapy for unresectable colorectal liver metastases: modern USA experience Int. J Radiat Oncol Biol Phys 65(2):412–425
Sailer SL (2000) Three dimensional conformal radiotherapy. In: Gunderson L, Tepper J (eds) Clinical radiation oncology. Churchhill Livingstone, Philadelphia, pp 236–255
Fox RA, Klemp PFB, Egan B et al (1991) Dose distribution following selective internal radiation therapy. Int J Radiat Oncol Biol Phys 21:463–467
Herfarth KK, Debus J, Lohr F et al (2001) Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 19:164–170
Gulec SA, Fong Y (2007) Yttrium 90 microspheres selective internal radiation treatment of hepatic colorectal metastases. Arch Surg 142(7):675–682
Dancey JE, Shepherd FA, Paul K (2000) Treatment of nonresectable hepatocellular carcinoma with intrahepatic Y90 microspheres. J Nucl Med 41(10):1673–1681
Jakobs TF, Hoffmann RT, Dehm,f chemtherapy–refractory colorectal cancer liver metastases. J Vasc Interv Radiol 19(8):1187–1195
Mancini R, Carpanese L, Cosimelli M et al (2006) A multicentric phase II clinical trial on intra-arterial hepatic radiotherapy with 90yttrium SIR–spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: rates. In Vivo 20(6A):711–714
Park JO, Lee SI, Song SY et al (2003) Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J Clin Oncol 33(10):533–537
Atassi B, Bangash A, Bahrani A et al (2008) Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics 28:81–99
Bienert M, McCook B, Carr B et al (2005) Y90 microsphere treatment of unresectable liver metastases: changes in (18)F-FDG ptake and tumour size on PET/CT. Eur J Nucl Med Mol Imaging 32:778–787
Wong C, Qing F, Savin M et al (2005) Reduction of metastatic load to liver after intraarterial hepatic yttrium-90 radioembolization as evaluated by [18F] fluorodeoxyglucose positron emission tomographic imaging. J Vasc Interv Radiol 16:1101–1106
Kwee TC, Takahara T, Ochiai R et al (2008) Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 18:1937–1952
Gray B, Van Hazel G, Hope M et al (2001) Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 12:1711–1720
Jakobs TF, Hoffmann RT, Schmitz A et al (2006) Treatment of extensive metastatic liver disease with intrahepatic yttrium 90 microspheres: toxicity assessment. Presented at SIR Annual Meeting, Toronto, Canada, 30 March–4 April
Van Hazel G, Price D, Bower G et al (2005) Selective internal radiation therapy (SIRT) plus systemic chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin: a phase I dose escalation study. Proceedings of ASCO GI Cancers Symposium 2005:133 (abstract)
Van Hazel G, Blackwell A, Andersonn J et al (2004) Randomized phase II trial of SIR-spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer J. Surg Oncol 88(2):75–85
Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA Jr, Kulik L, Geschwind JF, Murthy R, Rilling W, Liu D, Bester L, Bilbao JI, Kennedy AS, Omary RA, Salem R (2007) Radioembolization with 90Y microspheres:angiographic and technical considerations. Cardiovasc Interv Radiol 30(4):571–592
de Luis E, Bilbao JI, de Ciércoles JA et al (2008) In vivo evaluation of a new embolic spherical particle (HepaSphere) in a kidney animal model. Cardiovasc Interv Radiol 31(2):367–376
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cianni, R., Urigo, C., Notarianni, E. et al. Selective Internal Radiation Therapy with SIR-Spheres for the Treatment of Unresectable Colorectal Hepatic Metastases. Cardiovasc Intervent Radiol 32, 1179–1186 (2009). https://doi.org/10.1007/s00270-009-9658-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9658-8