Abstract
The uncommon presentation of an arterioportal fistula (APF) involving the superior mesenteric artery (SMA) associated with a pseudoaneurysm represents a therapeutic challenge. We present the case of a 24-year-old female admitted to the hospital after multiple gunshot wounds to the abdomen; the patient underwent multiple surgeries and, in the process, developed a SMA pseudoaneurysm and fistula. The vascular interventional radiology team was consulted for treatment of the pseudoaneurysm and fistula. A covered stent was inserted percutaneously to exclude the APF and the pseudoaneurysm in a single procedure. The patient returned to our service after 21 months for a follow-up CT scan, which demonstrated the stent and the distal vasculature to be patent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extrahepatic arterioportal fistula (APF) and pseudoaneurysms are rare but recognized complications of abdominal surgery or trauma to the visceral arteries [1]. There have been reports of these two types of lesions in the literature, but the combination of both involving the superior mesenteric artery (SMA) has not been reported. Although fistulas and pseudoaneurysms can be treated by surgery, endovascular management of these problems has offered an attractive alternative in some cases. We present a case of APF and a pseudoaneurysm from the proximal branch of the SMA, which was managed with a single covered stent.
Case Report
A 24-year-old woman who sustained multiple gunshot wounds to the abdomen presented to the emergency room at our institution with marked tachycardia and a tender abdomen. The patient was taken to the operating room for an emergency exploratory laparatomy. Due to her critical condition and hemodynamic instability, the patient was taken to the operating room without any imaging studies.
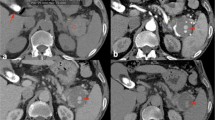
The patient was found to have lacerations involving the stomach, pancreas, duodenum, inferior vena cava, and right kidney and a laceration involving a branch of the superior mesenteric and splenic arteries. Surgical repair of all the lacerations was performed. The patient was coagulopathic at the end of the surgery. The abdomen was not closed and was packed. She was then transferred to the Trauma Intensive Care Unit and, 24 h hours later, underwent a second surgery, as colonic necrosis was identified. Resection of the necrotic intestinal segment was performed, along with pyloric exclusion surgery. After 4 days the patient developed high-grade fever (103°F) with chills and tachycardia, and a third surgery was performed at this time for colonic perforation and fecal peritonitis. The patient underwent a hemicolectomy and aggressive irrigation of the peritoneal cavity was done. On postoperative day 1, after the third surgical procedure, the patient was noted to have a palpable thrill medial to the second portion of the duodenum and liver on physical exam, suspicious for a mesenteric root arteriovenous fistula. The patient had undergone repair of a laceration of a branch of the SMA at the time of initial presentation, but the palpable thrill was a new finding and was thought to be secondary to iatrogenic injury. The patient underwent a duplex ultrasound study the next day, which demonstrated a pulsatile high-velocity waveform in the portal vein confirming the fistula. Following this diagnosis the patient had three more abdominal surgeries for washout and insertion of a vicryl mesh. One week following her last surgery the patient underwent CT of the abdomen with contrast, which demonstrated a 4 × 3-cm pseudoanuerysm from a branch of the SMA and portal vein opacification in the arterial phase of the study (Fig. 1), secondary to a fistulous communication between the SMA and the portal vein (Fig. 2). The patient was referred to the interventional radiology team for a diagnostic angiogram and possible intervention.
A selective SMA angiogram showed the pseudoaneurysm distal to the origin of the SMA, and the fistulous communication with the portal vein (Figs. 3–5). In an attempt to treat both problems with a single intervention, we elected to place a covered stent in the SMA exclude the pseudoaneurysm and a fistula. Attempts to place a 9-Fr Northstar Lumax Flex (Cook, Boomington, IN) guiding catheter past the origin of the SMA from the femoral approach were unsuccessful. A covered stent could not be placed and the procedure was terminated.
The vascular surgery team was consulted for a cutdown, to perform the procedure from an axillary approach. The next day, a left axillary artery cutdown was performed in the operating room and a 6-Fr vascular sheath was placed with fluoroscopic guidance provided by a C-arm (OEC 9800; GE Healthcare, Waukesha, WI). The SMA was catheterized with a 4-Fr cobra catheter (Cook) and a repeat angiogram was performed. The fistula and pseudoaneurym were identified and the vascular sheath and the cobra catheter were exchanged for a 9-Fr Northstar Lumax Flex (Cook) guiding catheter and a 10-Fr sheath. The diameter of the normal SMA measured 6.4 mm. A 7 mm × 5-cm Viabahn (W. L. Gore & Associates. Flagstaff, AZ) covered stent was advanced over a Rosen wire and was deployed across the pseudoaneurysm and fistula. A postprocedure angiogram through the guiding catheter demonstrated complete exclusion of the fistula and pseudoaneurysm, with normal distal SMA branches. Therefore, balloon dilatation was not performed (Fig. 6). Postprocedure hospital course was uncomplicated. The patient received Vancomycin and Levaquin and was discharged 19 days after the procedure to a rehabilitation center where she had a full recovery. She remained asymptomatic at the 12-month follow-up visit.
The patient returned to our service 21 months after the procedure for a follow-up. Contrast-enhanced CT of the abdomen and pelvis with three-dimensional reconstruction of the mesenteric vessles demonstrated a patent stent in the SMA, with normal distal branch vessels and no evidence of the pseudoaneurysm or the fistula (Figs. 7 and 8).
Discussion
Injuries to the mesenteric vessels can be lethal and challenging complications of penetrating and blunt abdominal injury [2]. An incidence of 0.09% has been reported, mostly due to penetrating trauma in young males, with a mortality rate of 39–77% for those presenting with proximal mesenteric artery injuries [3]. These injuries pose a surgical challenge that tests the skills of even the most experienced trauma surgeons. Exsanguinating hemorrhage accounts for the majority of early deaths. Late deaths usually occur secondary to sepsis, multisystem organ failure, and the sequelae of ischemic bowel, in those who survive their initial surgical intervention [4]. Outcomes have been correlated with the presence of shock on admission [4], anatomic location of injury [5], presence of infracted bowel [5], method of surgical repair [4], and number of associated injuries [4].
Injury to the vessel, either after blunt abdominal trauma or iatrogenically, can result in the formation of a hematoma around it. This can develop into a false aneurysm which can subsequently communicate with an adjacent injured artery or vein, thus giving rise to an arteriovenous fistula.
The symptoms associated with APFs depend on the location of the fistula, the amount of blood shunted, and the resistance of the liver to the increased mesenteric and portal venous flow [6]. Posttraumatic superior mesenteric arteriovenous fistulas (SMAVFs) have rarely been described, with 40 cases reported in the literature over the last 60 years [1]. Early recognition and treatment can prevent the late sequelae of portal hypertension. Most patients, have a delayed presentation, with weight loss, abdominal pain, signs of right heart failure, pleural effusion, signs of portal hypertension including ascites, and mesenteric ischemia [3]. The most consistent finding is an abdominal thrill that can be palpated in the periumbilical or epigastric region just left of the midline with a systolic accentuation on auscultation [7].
Diagnosis can be established by combined B-mode and color Doppler examination or CT angiography.
Treatment options include open and endovascular approaches. The endovascular treatment options include embolization and covered stent placement. The challenge of embolization lies in the specific anatomic features of the fistula and pseudoaneurysm. Coil migration and nontarget embolization with occlusion of mesenteric arterial branches are potential complications. Embolization was not believed to be appropriate in this case due to the size of the fistula and the pseudoaneurysm [8]. Therefore, we elected to use a covered stent to exclude both the fistula and the pseudoaneurysm [9].
Various handmade and commercial stent grafts have been described in the literature [10]. We used the Viabahn (W. L. Gore and Associates, Inc.,) covered stent, which is a nitinol metal stent covered with polytetrafluroethylene (ePTFE) and has been approved by the FDA for use in the superficial femoral artery and for tracheobronchial strictures. These covered stents are nonparamagnetic, crush resistant, and self-expandable with negligible foreshortening and they have a high elasticity. They deploy from distal to proximal end and cannot be repositioned or retrieved once deployment starts. The manufacturer recommends using a covered stent that is 1 mm larger in size than the adjacent normal artery. It is not necessary to balloon dilate the stent after deployment, but this can be performed if there is unsatisfactory expansion or a persistent fistulous communication.
Conclusion
Although long-term data for use of covered stents in visceral arteries is extremely limited, endovascular treatment of traumatic APF with pseudoaneurysm is a technically feasible and effective alternative to surgery. Long-term follow-up and a wider acceptance of this procedure could provide enough evidence to offer an alternative to open surgery in the management of this condition. However, randomized controlled trials must be performed to standardize the placement of covered stents as a treatment option in the management of this condition.
References
Chiriano J, Jr Abou-Zamzam AM, Teruya TH et al (2005) Delayed development of a traumatic superior mesenteric artery fistula following multiple gunshot wounds to the abdomen. Ann Vasc Surg 19(4):470–473
Asensio J, Forno W, Roldan G et al (2002) Visceral vascular injuries. Surg Clin North Am 82:1–20
Asensio J, Britt L, Borzotta A et al (2001) Multiinstitutional experience with the management of superior mesenteric artery injuries. J Am Coll Surg 193:354–365
Asensio JA, Berne JD, Chahwan S et al (1999) Traumatic injury to the superior mesenteric artery. Am J Surg 178:235–239
Fullen WD, Hunt J, Altemeier WA (1972) The clinical spectrum of penetrating injury to the superior mesenteric arterial circulation. J Trauma 12:656–664
Puglionisi A, Di Giovanni V, Snider F, Camilli S, Cina G (1980) A propos d’un cas de fistule arte´rio-veineuse he´patico-portale. Proble`mes de physiopathologie et clinique et revue de la litte´rature. J Chir 117:607–619
Radonic V, Baric D, Petricevic D et al (1995) Advances in diagnostics and successful repair of proximal posttraumatic superior mesenteric arteriovenous fistula. J Trauma 38:305–312
Cowan S, Kahn MB, Bonn J et al (2002) Superior mesenteric artery pseudoaneurysm successfully treated with polytetrafluoroethylene covered stent. J Vasc Surg 35:805–807
Shih F, Sy-jau W, Keh-jong D et al (1994) Successful management of traumatic mesenteric arteriovenous fistula after failure of steel coil embolization: case report. J Trauma 37:682–686
Appel N, Duncan JR, Schuerer DJ (2003) Percutaneous stent-graft treatment of superior mesenteric and internal iliac artery pseudo aneurysms. J Vasc Interv Radiol 14(7):917–922
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narayanan, G., Mohin, G., Barbery, K. et al. Endovascular Management of Superior Mesenteric Artery Pseudoaneurysm and Fistula. Cardiovasc Intervent Radiol 31, 1239–1243 (2008). https://doi.org/10.1007/s00270-008-9354-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-008-9354-0