Abstract
The purpose of this study was to retrospectively determine the safety and effectiveness of percutaneous cryoablation, monitored with computed tomography (CT) and ultrasonographic (US) guidance, for the treatment of hepatocellular carcinoma (HCC). Four patients with small HCCs underwent one percutaneous cryoablation treatment session monitored with CT and US guidance. All patients underwent pretreatment blood chemistry testing and imaging evaluation. We treated lesions with simultaneous insertion of multiple 17-G cryoprobes (two or three) and defined technical success when the extension of a visible iceball was beyond 5 mm from the tumor margin. Intralesional enhancement or tumoral size increase was defined as local progression compared with that on images obtained immediately after ablation. We evaluated complications and follow-up (at 1, 3, and 6 months). All patients survived without short- or long-term complications. Cryoablation was technically successful in all patients at the end of the procedure. During follow-up two patients developed disease recurrence. One patient developed local tumor progression on the margin of the lesion; the other, a new HCC. In the case of local tumor progression a new elevation of α-fetoprotein (αFP) levels occurred at first follow-up control. In the other case levels of αFP remained stable during the first 3 months after the procedure, then demonstrated a progressive increase in αFP levels beginning at the fourth month, without tumor evidence during CT control at 3 months. We conclude that percutaneous cryotherapy with US guidance and CT monitoring is a feasible, safe, and effective for treatment of HCC. If local ablative procedures of hepatic lesions are to be performed, percutaneous cryoablation, not laparotomic, should be discussed as an alternative therapeutic measure. Longer follow-up should provide proof of the effectiveness of this technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide, with a 5.4% cancer mortality rate [1, 2]. Median age at diagnosis is 50-60 years, with the major incidence in males. Hepatitis B virus (HBV) and C virus (HCV) are the main causes leading to development of HCC. Other common risk factors are ethanol and aflatossina. Cirrhosis is a precancerous condition with an annual rate of malignant transformation between 2% [3]and 5% [4]. Patients with combined HBV and HCV infection have the greatest risk.
Surgical resection is still maintained as the treatment of choice but it is possible in only 30% of cases [5, 6]. Liver resection for curative treatment has been limited by the number and size of lesions, the presence of tumor in unresectable locations, the presence of tumor in multiple segments, hepatic failure, and underlying conditions or illness that render carcinoma inoperable. Transplant is the best therapy in the treatment of HCC but it is limited by organ availability. Various targeted ablation techniques have been introduced in attempts to overcome the limitations of hepatic resection and to increase the number of patients eligible for tumor removal or tumor ablation. These locoregional treatments include ethanol injection, radiofrequency ablation (RFA), laser photocoagulation, high-intensity focused ultrasound (US), and cryoablation [7–11].
Cryoablation, also known as cryotherapy, is a technique based on in situ freezing and devitalization of tissues, which can be applied and controlled precisely to produce a predictable zone of necrosis that will destroy the target lesion as well as an appropriate margin of surrounding tissue. Cryotherapy is a thermal ablation technique that has been used extensively in open surgical settings and, more recently, applied percutaneously to treat renal tumors [12].
Nevertheless, at present there are no studies on percutaneous cryoablation under US guidance monitored with computed tomography (CT) in the treatment of HCC, although the intraoperatory one is already used in the treatment of primary and secondary liver tumors. The most common technique for imaging interstitial cryotherapy is US but it has some limitations with regard to monitoring the freezing procedure. US can depict the effects of freezing in real time and accurately depict the zone of necrosis that cryoablation produces; however, because of shadowing behind its proximal edge, the circumference of the iceball cannot be seen on a single view [13]. Several experimental studies have proven the capability of magnetic resonance imaging (MRI) in imaging the extension of frozen tissue with a high accuracy and excellent contrast between the normal and the frozen liver tissue due to the extreme decrease in T2 relaxation time of frozen water [14]. CT, as a real-time monitoring device for cryoablation, has not been widely used.
The aim of our study was to retrospectively determine the safety and effectiveness of percutaneous cryoablation under US guidance monitored with CT for the treatment of small HCC (<3 cm) and to evaluate short-term outcome.
Materials and Methods
From October 2006 to May 2007 four patients (three men and one women; average age, 56 years), each with solitary HCC, underwent percutaneous cryoablation with US guidance and CT monitoring. The diagnosis of HCC was confirmed in all patients in accordance with the Barcelona guidelines [15]. The diameters of the tumors were ≤3 cm (range, 1.8 to 3 cm): one lesion was localized in segment III, one lesion in segment IV, and two in segment VIII.
Patients were selected if surgery was contraindicated or refused, their chronic liver disease was well compensated (three cases were in Child’s Class A and one in Class B), portal thrombosis and extrahepatic spread were absent, and no other form of treatment had been administered over the previous 6 months (Table 1). One patient was subjected to surgical resection of HCC 2 years previously and to RFA 1 year previously. In addition, a major selection criterion was nodule site: the margin of the nodule should be at least 1 cm away from vital structures such as gallbladder, hepatic hilum, and hepatic veins to avoid thermal damage.
The treatment was approved by our institutional review board, and informed consent was obtained from all patients before the procedure.
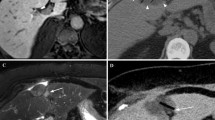
All patients underwent pretreatment blood chemistry testing and imaging evaluation. We considered total and fractionated bilirubin, Quick’s time, INR, albumin, platelets, ammonia, α-fetoprotein (αFP), and ferritin and hepatitic markers. We obtained an ECG for each patient. Before the cryoablation procedure all patients also underwent upper abdominal US imaging (ATL HD 5000; Philips, Best, Netherlands) and 64-multislice CT scan (LightSpeed 64 CT; GE Medical Systems, Milwaukee, WI, USA) with the three-phase technique (30, 65, and 180 s) following intravenous injection of a 120 ml bolus of iodinated contrast medium (300 mgI/ml; Ultravist, Schering) and a 20 ml bolus of physiologic solution (Figs. 1 and 2). Scanning parameters were 1 s gantry rotation time, 1.25 mm collimation with the possibility of retroreconstruction at 0.6 mm, 360 mA, and 120 kV.
Contrast medium was injected at a rate of 2.5–3 ml/s through an 18 G intravenous catheter inserted in an antecubital vein. Each patient was treated after local injection of 10 ml lidocaine (2%). After sonographically determining the most favorable percutanous approach, we inserted two or three cryoprobes (17 G, 14 cm long; Galil Medical, Yokneam, Israel) into the tumor under US guidance and advanced the tip to reach the distal margin of the targeted lesion. The HCCs were well and simply visualized without the use of contrast media. Cryoprobe positions were determined according to tumor geometry and expected iceball size. CT was used to verify placement of the multiple cryoprobes (Fig. 3). Each cryoprobe was equipped with a thermocouple that monitored needle-tip temperature throughout freezing and thawing. A computer interface displayed needle-tip temperatures and could be used to input the desired temperature during the procedure. Heat exchange occurred along only a 4 cm long segment at the distal end of each cryoneedle. The system consisted of a computer workstation, a gas gauge, a gas distribution system, and accessories that included needle-like cryoprobes, temperature sensors, and a remote control device. It used a high-pressure cooling gas (argon), which achieved temperatures as low as −185°C at the tip of the needle probes. The argon was converted to cold low-pressure liquid using the joule effect. To thaw the tissues a high-pressure gas (helium) was converted to a warm low-pressure gas.
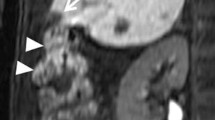
Each lesion received a treatment cycle of freezing (−60°C), heating (up to 70°C), and refreezing to avoid tumor cell seeding and the duration of the procedure was based on growth of the iceball relative to the tumor (mean, 15 min; range, 10–18 min). Limited unenhanced CT scans were obtained approximately every 3 min during the first freezing time using 1.25 mm collimation to monitor growth of the iceball. CT was used to accurately monitor iceball size and location and help predict subsequent cell death (Figs. 4 and 5) [16, 17]. We used a single cycle (formed by freezing-heating-freezing) because we wanted to demonstrate the efficacy of single and short-time treatment in accordance with in vitro studies by Tacke et al. who observed no differences in maximum diameter among three freezing cycles that were performed at different locations in the same liver with direct visual measurement [14].
Because complete cell death occurs approximately 3 mm inside the edge of the iceball, the goal was to extend the iceball 5 mm beyond the tumor margin during freezing time [18, 19].
Treatment success was defined as extension of the iceball 5 mm beyond the tumor margin and absence of arterial contrast enhancement during postprocedural CT control after cryoprobe removal (Fig. 6). Once complete tumor ablation had been achieved, all patients were included in the follow-up program. At 1, 3, and 6 months patients underwent alkaline phosphatase, total and fractionated bilirubin, transaminases, γ-glutamyl transpeptidase (γGT), carcinoembryonic antigen (CEA), albumin, Quick’s time and INR, complete blood count (CBC), and platelet and αFP testing. The Child-Pugh class was reviewed. At 1, 3, and 6 months patients underwent triphasic CT for evaluation of the ablation zone and detection of local tumor progression and/or new HCC lesions. We distinguished two types of recurrence disease: local tumor progression and new HCC lesions. The first was the presence of neoplastic tissue within the same liver segment at the margin of a treated HCC nodule and the second was the development of nodules in a different liver segment.
All short- and medium-term complications were considered.
Results
Our initial experience was treating four patients with HCC. The maximum diameter of the four lesions ranged from 1.8 to 3 cm. Cryoablation was technically successful in all patients at the end of the procedure. No repeat treatment was needed because there was no contrast enhancement in postprocedural CT control, as the diameters of the iceballs obtained ranged from 3 to 4 cm with an extension of the iceball 5 mm beyond the tumor margin in all cases. Each HCC received a treatment cycle of freezing, heating, and refreezing; and the duration of the procedure was based on growth of the iceball relative to the tumor (mean, 15 min; range, 10–18 min). The number of cryoprobes used during ablation was two or three on the basis of tumor diameter. The iceball Hounsfield unit (HU) value was 18–22, obtained with a region of interest of 2 mm localized in the area between the frozen/unfrozen edge and the cryoprobe.
No deaths or major complications (no hemorrage found in any case) occurred during the procedures, and no cases of neoplastic seeding have been observed to date. During follow-up two patients developed disease recurrence. One patient developed local tumor progression visualized as perilesional contrast enhancement at first CT follow-up control at 1 month (Fig. 7). The same patient was subjected to surgical resection of another HCC lesion 2 years previously and to RFA 1 year previously. The second patient developed a new HCC that was detected 6 months after the procedure. No local tumor or new lesions were found in the other 2 patients.
Two weeks after treatment αFP levels returned to normal in all patients. In the case of local tumor progression a new elevation of αFP levels occurred at first follow-up control (1 month). In the other (the patient with a new HCC lesion), levels of αFP remained stable during the first 3 months after the procedure, demonstrating a progressive increase in αFP levels beginning at the fourth month without tumor evidence during CT control at 3 months. We did not observe variations of other parameters.
Fever lasting 1–4 days was observed in two patients, and pain lasting 12–36 h after treatment was reported by one patient. Administration of a painkiller was necessary in the same case (paracetamol per os, 500 mg, four per day, for 2 days). During follow-up we observed no cutaneous or abdominal wall tumor implantations (seeding) along the needle tract either clinically or on CT.
Discussion
In recent years, sonographically guided percutaneous ablative therapies have been proposed as valid alternatives to surgery for treatment of HCC in patients with cirrhosis, which represents the major risk factor for development of the tumor [20]. Various targeted ablation techniques have been introduced in attempts to overcome the limitations of hepatic resection (number and size of HCCs, presence of tumor in unresectable locations, presence of tumor in multiple segments, underlying conditions or illnesses that render carcinoma inoperable), increasing the number of patients eligible for tumor removal, or to improve survival. Although some of these regional treatments have shown promise, each has considerable limitations.
Percutaneous ethanol injection treatment is limitated by the difficulty of predicting the volume of necrosis achievable and the possible serious collateral effects of ethanol diffusion into nontumorous liver tissue. For these reasons, alternative percutaneous ablative procedures have been proposed for the treatment of liver tumors. RFA has been proven in several studies to be a reliable method with which to treat HCC [21]. Nowadays RFA has an important role in the treatment of small HCCs [22, 23]. Mahnken et al. demonstrated that MR-guided RFA of hepatic malignancies (primary and secondary) is technically feasible and safe. So it can be advantageous in locations considered unfavorable for CT-guided puncture or in patients in which iodinated contrast material is contraindicated [24]. RFA involves a rapidly alternating current to cause tissue heating and, ultimately, cell death. Although bleeding is not common in patients who undergo this procedure, questions remain about the reliability of RFA to cause cell death within a target zone [8, 25]. This same limitation applies to laser photocoagulation and to high-intensity focused US [9, 10]. In addition, the use of real-time imaging guidance for RFA, laser photocoagulation, and high-intensity focused US to accurately predict the degree of tissue necrosis has been a problem [26].
Cryoablation is accepted because it produces ice balls of consistent size and shape, and it reliably causes cell death within the cryolesion [27]. However, the technology has been traditionally limited to intraoperative use because of the large probes, which can cause serious bleeding when removed [28]. Cryotherapy is believed to kill cells by several mechanisms including intracellular ice formation, solute-solvent shifts that cause cell dehydration and rupture, and small-vessel obliteration with resulting hypoxia [11]. Moreover, another big advantage of cryoablation is the sharp margin between destroyed and intact liver tissue. Temperatures below −40° to −50°C are assumed to be necessary to ensure lethal freezing injury to neoplastic tissue. Complete cell death occurs when there is a perfect location match between the target lesion and the cryolesion volume colder than −40°C. In clinical settings such a perfect match is difficult or impossible to accomplish. CT can define the complete three-dimensional boundary of an ice ball by the signature change in HU value from unfrozen to completely frozen tissue. The internal architecture of the frozen tissue can also be imaged and the point-to-point relative HU values are maintained in the frozen volume at different average values. However, no discernible relation exists between the temperature in completely frozen tissue and its HU value in the temperature range investigated. At present there are no studies on the correlation between tissue temperature and HU in vivo, but only on in vitro models of inhomogeneous gelatin-calf liver phantom that permits obtaining isotherm surfaces correlated with density [29, 30].
Percutaneous cryoablation, comparated with RFA, has three theoretical important advantages in the treatment of HCC. First, and most important, CT can help accurately monitor the cryoablation procedures and supplies three-dimensional views of the ice ball as it develops [17]. Second, multiple cryoprobes can be used simultaneously in the treatment of large tumors. Potentially cryoablation can successfully treat tumors >3 cm in diameter; there are no studies that show applications of this technique in HCC >3 cm, but a recent study demonstrates that percutanous cryoablation with US guidance and CT monitoring appears to be safe and effective for the treatment of solid renal masses (>3 cm) [12]. However, the 1 cm rim zone does not seem to ensure temperatures of −40°C in the whole tumor volume except for small tumors like the lesions that we treated [30]. Third, direct monitoring of the cryoablation and probe-specific control of the cryoablation system permits regulation of individual cryoprobes to slow or stop the freezing process if the growing ice ball approaches a vital structure.
Percutaneous cryoablation is limited by the potential for collateral damage to organs proximal to the liver. During open cryoablation, the intestine and the diaphragm are protected with insulating material. The inability to protect these organs will limit the use of percutaneous cryoablation to lesions that are deep in the liver parenchyma or remote from vital extrahepatic structures. Nevertheless, when necessary, water can be percutaneously injected to shift critical structures (i.e., colon) away from the area being treated. This approach has already been used to manipulate vital adjacent structures to allow cryoablation in renal cell carcinoma [31].
Percutaneous cryoablation has two advantages over the laparoscopic and laparotomic approach. The first is less invasive than the corresponding laparoscopic and laparotomic methods, which can determine coagulation profile disorders leading to DIC, liver failure, myoglobinurea, and other side effects. Most important, the ablation zone can be carefully monitored with intermittent CT. With intraoperative cryoablation, US guidance is used, and the acoustic shadow of the ice ball precludes the imaging of deep tissue. Thus only the leading edge of the ice ball is seen. Moreover, percutaneous cryoablation can also be performed with the patient receiving conscious sedation instead of general anesthesia. Another important advantage is the reduced time that the patient spends in the hospital. This effects an important consequent reduction in hospitalization costs.
We are aware of a single case in which percutaneous cryoablation was performed in a patient who was not a surgical candidate (G. M. Onik, oral communication, 1998). The procedure was guided with US, without bleeding or other complications, and the patient was rapidly discharged from the hospital in excellent condition. We did not find other works on HCC percutaneous cryoablation. Moreover, we used 17 G needles for the procedure. This choice can theoretically reduce local complications and make percutaneous cryoablation safer and more simple than other local techniques that use needles of bigger diameter [17].
An inherent limitation of percutaneous cryoablation compared with the laparoscopic method is the potential bleeding, which cannot be controlled directly without intra-arterial access and fluoroscopy. In contrast, bleeding may be controlled directly during laparoscopic surgery, but this advantage must be viewed in the context of the higher risks associated with a more invasive procedure.
Imaging during the cryotherapy procedure is possible by US, CT, and MR. US is widely availabe and enables online monitoring of the freezing process and good differentiation between frozen and unfrozen tissue. Incomplete imaging of the entire ice ball, low spatial resolution in deeper structures, and operator dependency are disadvantageous. CT is operator independent; the ice/tissue contrast depends on the slice thickness, and the use of ionizing irradiation may be considered less favorable also for artifacts caused by the metallic cryoprobes. CT could permit complete imaging of the freezing process by use of a metal-free cryoprobe without the problem of metal artifacts [32].
MR provides excellent monitoring of the cryosurgical process. The cryolesion edge is clearly defined due to signal void (T2-weighted images). Three-dimensional MR estimation of cryolesion temperature and display of the tumor within the area of signal void may further improve MR monitoring of the cryoablative procedure. Some studies demonstrate that MR can distinguish the area of necrosis 24 h after the procedure, while US and CT do not accurately reflect the zone of necrosis [33]. Nevertheless MR-guided percutaneous cryotherapy shows some disadvantages: the use of general anesthesia, long procedure time (3–6 h), use of MR-compatible cryoablation materials, and high costs of this technique. Moreover, gradient echo sequences generally understimated the ice-ball diameter by 15% [14].
Our initial experience showed technical success in all four patients. The interventional procedure was well tolerated by all patients; neither major complications nor procedure-related deaths occurred, including severe liver decompensation.
We observed one case of local tumor progression and one new HCC. A study on disease recurrence highlighted that recurrence was frequent in patients with nonresectable HCC treated with RFA [34]. However, the biological features of this tumor (ab initio organ pathology) make it clear that disease recurrence is not directly related to the procedure itself. This fact is in accord with our results: one patient developed local tumor progression visualized as perilesional contrast enhancement at 1-month CT follow-up control. The same patient was subjected to surgical resection of HCC 2 years previously and to RFA 1 year previously. In clinical settings a perfect match between the target lesion and the cryolesion volume colder than −40°C is difficult or impossible to accomplish. In fact during cryoablation multiple probes may be used simultaneously, and in such situations the temperature profile may be different from that in experimental in vitro studies. Most likely the volume of the ice ball did miss the tumor margin or a vessel nearby provided warming of nested tumor cells nearby. Cryolesion temperatures may also be influenced by the type of delivery system, the cryogen used, and the cryoprobe size. Tumor tissue has been demonstrated to be more resistant to freezing than normal liver tissue and these physical differences may influence temperature distribution within cryolesions of normal and malignant tissue [30]. Perhaps the development of the ice ball could determine an increase in the tissue volume that could shift the tumoral margin to a distance <3 mm under the ice-ball edge. Seifert et al. estimated the incidence of tumor persistence as local recurrence at the site of ablation between 5 and 60% [35].
During follow-up another patient developed disease recurrence as a new HCC. This event may be due in part to the natural history of HCC, which has a well-known high tendency to recur, and in part to the understaging of the liver tumor.
An important limitation of our study concerns the possibility of differentiation between technical failure and local tumor progression. We defined technical success as the extension of the ice ball 5 mm beyond the tumor margin during the ablation procedure. Such technical success may be seen only in retrospect by observing the location of enhancement on follow-up images. We can consider marginal enhancement of the ablation site at short-term follow-up (<6 months) as being equivalent to suboptimal marginal treatment (technical failure), whereas central enhancement or tumor enlargement is indicative of true local tumor progression.
All the techniques used to monitoring cryoablation cannot visualize the tumoricidal part of the ice ball, and we can only base conclusions on experimental values obtained in vitro that demonstrate a relation between density/signal intensity and tissue temperature.
In conclusion, percutaneous cryoablation with US guidance and CT monitoring appears to be safe and effective for the treatment of HCC. Our initial experience has no statistical value. Therefore our results cannot be compared with other studies on the therapeutic use of other interventional or intraoperative techniques. Larger studies with longer follow-up of the treated patients are required to provide a better definition of the indications and limits of cryoablation of HCC arising in cirrhosis and to compare this innovative technique with RFA, which is a procedure validated as a percutaneous ablative method for treatment of small HCCs.
References
Colombo M, Sangiovanni A (2003) Etiology,natural history and treatment of hepatocellular carcinoma. Antiviral Res 60:145–150
Parkin DM, Bray F, Ferley J et al (2001) Estimating the world cancer burden: Globocan 2000. Int J Cancer 94:153–156
Colombo M, de Franchis R, Del Ninno E et al (1991) Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 325:675–680
Bolondi L (2003) Screening for hepatocellular carcinoma in cirrhosis. J Hepatol 39:1076–1084
Levin B, Amos C (1995) Therapy for unresectable hepatocellular carcinoma. N Engl J Med 332:1294–1296
Francica G, Marone G (1999) Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a “cooled-tip needle.” A preliminary clinical experience. Eur J Ultrasound 9(2):145–153
Raviumar TS (1996) Interstitial therapies for liver tumors. Surg Oncol Clin N Am 5:365–377
Livraghi T, Goldberg SN, Monti F et al (1997) Saline-enhanced radio-frequency tissue ablation in treatment of liver metastases. Radiology 202:205–210
Amin Z, Donald JJ, Masters A et al (1993) Hepatic metastases: interstitial laser photocoagulation with real time US monitoring and dynamic CT evaluation of treatment. Radiology 187:339–347
Yang R, Sanghvi NT, Rescorla FJ et al (1993) Liver cancer ablation with extracorporeal high-intensity focused ultrasound. Eur Urol 23:17–22
Kane RA (1993) Ultrasound-guided hepatic cryosurgery for tumor ablation. Semin Interv Radiol 10:132–142
Atwell TD, Farrell MA, Callstrom MR et al (2007) Percutaneous cryoablation of 40 solid renal tumors with US guidance and CT monitoring: initial experience. Radiology 243:276–283
Weber SM, Lee FT, Warner TF et al (1998) Hepatic cryoablation: US monitoring of extent of necrosis in normal pig liver. Radiology 207:73–77
Tacke J, Adam G, Haage P et al (2001) MR-guided percutaneous cryotherapy of the liver: in vivo evaluation with istologic correlation in a animal model. J Magn Reson Imag 13:50–56
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the study of the liver. J Hepatol 35:421–430
Sandison GA, Loye MP, Rewcastle JC et al (1998) X-ray CT monitoring of iceball growth and thermal distribution during cryosurgery. Phys Med Biol 43:3309–3324
Lee FT Jr, Chosy SG, Littrup PJ et al (1999) CT monitoring percutaneous in a pig liver model: pilot study. Radiology 211:687–692
Campbell SC, Krishnamurthi V, Chow G et al (1998) Renal cryosurgery: experimental evaluation of treatment parameters. Urology 52:29–33
Chosy SG, Nakada SY, Lee FT Jr et al (1998) Monitoring renal cryosurgery: predictors of tissue necrosis in swine. J Urol 159:1370–1374
Livraghi T (2001) Guidelines for treatment of liver cancer. Eur J Ultrasound 13:167–176
McGahan JP, Dodd GD 3rd (2001) Radiofrequency ablation of the liver: current status. AJR176:3–16
Buscarini L, Buscarini E, Di Stasi M et al (2001) Percutaneous radiofrequency ablation of small Hepatocellular carcinoma: long-term results. Eur Radiol 11:914–921
Lencioni RA, Allgaier HP, Cioni D et al (2003) Small hepatocellular carcinoma in cirrhosis: randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 228:235–240
Mahnken HD, Buecker A, Spuentrup E et al (2004) MR-guided radiofrequency ablation of hepatic malignancies at 1.5T: initial results. J Magn Reson Imag 19:342–348
Rossi S, Di Stasi M, Buscarini E et al (1996) Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR 167:759–768
Adams JB, Moore RG, Anderson JH et al (1996) High-intensity focused ultrasound ablation of rabbit kidney tumors. J Endourol 10:71–75
Ravikumar TS (1996) The role of cryotherapy in the management of patients with liver tumors. Adv Surg 30:281–291
Lee FT Jr, Mahvi DM, Chosy SG et al (1997) Hepatic cryosurgery with intraoperative US guidance. Radiology 202:624–632
Sandison GA, Loye MP, Rewcastle JC et al (1998) X-ray CT monitoring of iceball growth and thermal distribution during cryosurgery. Phys Med Biol 43:3309–3324
Mala T, Samset E, Aurdal L et al (2001) Magnetic resonance imaging-estimated three dimensional temperature distribution in liver cryolesion: a study of cryolesion characteristics assumed necessary for tumor ablation. Cryobiology 43:268–275
Farrel MA, Charboneau JW, Callstrom MR et al (2003) Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR 181:1315–1317
Wei J, Sandison GA, Chen L et al (2002) X-ray CT high-density artifact suppression in cryosurgery. Phys Med Biol 47(24):N319–N326
Silverman SG, Tuncali K, vanSonnenberg E et al (2005) Renal tumors: MR imaging-guided percutaneous cryotherapy-initial experience in 23 patients. Radiology 230:716–724
Harrison LE, Koneru B, Baramipour P et al (2003) Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg 197: 759–764
Seifert JK, Junginger T (2004) Cryotherapy for liver tumours: current status, perspectives, clinical results, and review of literature. Technol Cancer Res Treat 3:151–163
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orlacchio, A., Bazzocchi, G., Pastorelli, D. et al. Percutaneous Cryoablation of Small Hepatocellular Carcinoma with US Guidance and CT Monitoring: Initial Experience. Cardiovasc Intervent Radiol 31, 587–594 (2008). https://doi.org/10.1007/s00270-008-9293-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-008-9293-9