Abstract
Purpose
To evaluate the feasibility of using contrast-enhanced ultrasound (CEUS) during uterine artery embolization (UAE) in order to define the correct end-point of embolization with complete devascularization of all fibroids.
Methods
In this prospective study of 10 consecutive women undergoing UAE, CEUS was performed in the angiographic suite during embolization. When the angiographic end-point, defined as the “pruned-tree” appearance of the uterine arteries was reached, CEUS was performed while the angiographic catheters to both uterine arteries were kept in place. The decision whether or not to continue the embolization was based on the findings at CEUS. The results of CEUS were compared with those of contrast-enhanced magnetic resonance imaging (MRI) 1 day as well as 3 months following UAE.
Results
CEUS was successfully performed in all women. In 4 cases injection of particles was continued based on the findings at CEUS despite angiographically complete embolization. CEUS imaging at completion of UAE correlated well with the findings at MRI.
Conclusion
The use of CEUS during UAE is feasible and may increase the quality of UAE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The clinical success of uterine artery embolization (UAE) for symptomatic fibroids depends on the complete devascularization of all fibroids [1]. The correlating angiographic end-point, however, is a matter of subjective interpretation of angiograms and seems to depend on the type of particles used [2]. Contrast enhancement in fibroids at MRI studies 1 day and 33 months after UAE seems to predict clinical outcome. In cases of remnant contrast enhancement in fibroids after UAE, most investigators advise informing the patient about the probability of clinical failure and wait for clinical outcome before repeat UAE.
It has been shown that contrast-enhanced ultrasound (CEUS) is a very sensitive technique, capable of demonstrating even a low degree of perfusion [3–5] The use of CEUS during UAE has been described by Marret et al. in a case report published in 2004 [6].
The aim of our pilot study was to determine whether the use of CEUS is feasible during UAE and whether the additional information obtained by CEUS could possibly secure complete devascularization of fibroids before completion of the embolization procedure.
Materials and Methods
Ten women with symptomatic fibroids, scheduled for UAE, were consecutively included in this prospective study; their mean age was 38.7 years (range 27–51 years). Five patients had previously undergone surgical treatment: myomectomy in 4 patients and cesarean section in 1. All patients suffered from either metrorrhagia, urinary frequency, pelvic pain, or a combination of symptoms.
MRI studies using a 1.5 T unit (Magnetom Vision Plus, Siemens, Erlangen, Germany) were performed before the procedure as well as 1 day and 3 months after the procedure. Each examination included sagittal and axial T2-weighted turbo spin-echo (TSE) images as well as sagittal contrast-enhanced fat-suppressed T1-weighted images. For contrast enhancement, 0.1 mmol/kg body weight gadopentetate dimeglumine (Magnevist, Schering, Berlin, Germany) was injected intravenously. Six women had fewer than 5 fibroids, 3 women had 6–10 fibroids, and 1 woman had more than 10 fibroids.
Immediately prior to embolization, a transabdominal ultrasound examination was performed using an Acuson Sequoia 512 system (Siemens, Erlangen, Germany) with a 4C1 curved-array probe. We registered whether or not the entire uterus was visible.
For evaluation of fibroid vascularization 2.4 ml of ultrasound contrast agent (SonoVue, Bracco, Milano, Italy) were administered intravenously followed by a flush of 5 ml saline solution. The contrast studies were carried out using contrast-specific software (Cadence) with low mechanical index and pulse inversion technique. The contrast studies were stored as multiple video clips, each of 10 sec duration, for a total of at least 2 min after the injection of contrast medium. During the intraprocedural ultrasound examinations particular attention was paid to scanning the whole uterus during the arterial phase of contrast enhancement in order to depict any residual arterial flow in the fibroids. The intraprocedural ultrasound video clips were evaluated by visual assessment of fibroid tissue contrast enhancement after ultrasound contrast agent (USCA) injection.
In 9 patients, embolization was carried out by bilateral access with 4 Fr introducers; in the first patient only a unilateral femoral access was used. In all patients bilateral embolization of the uterine arteries was carried out using microcatheters. In 1 patient there was collateral blood supply to the fibroids from an ovarian artery which, however, was too small for selective catheterization and embolization. The embolic agent used was microspheres sized between 500 and 900 μm (Embosphere microspheres, Biosphere Medical, Roissy, France), injected simultaneously on both sides. The angiographic end-point was occlusion of the perifibroid arterial plexus with preservation of slow, antegrade flow in the uterine artery as described by Pelage et al. [7] (“pruned-tree” appearance) [8]. Whenever angiography indicated a potential end-point for injection of particles, a repeat angiography was performed after a 10 min delay, followed by a CEUS examination.
When the angiographic end-point was reached, we repeated the CEUS examination without removing the angiographic catheters. If there was complete lack of contrast enhancement of fibroids at CEUS, the procedure was ended. If there was any remnant contrast enhancement in fibroids at CEUS, and angiography still showed antegrade flow in the uterine arteries, we continued injection of particles. If there was remnant contrast enhancement in fibroids but complete cessation of flow in the uterine arteries, aortography was performed to depict any collaterals from the ovarian arteries. If these were present and accessible for embolization, the procedure was continued by either superselective distal catheterization and embolization of the ovarian arteries, or injection of larger particles (900–1200 μm) with the catheter placed in the trunk of the ovarian arteries.
If further embolization was performed, CEUS examinations were repeated until cessation of fibroid enhancement or until embolization was no longer technically possible. Again, a delay of 10 min between the last injection of particles and potentially final imaging by angiography and CEUS was observed.
Postprocedural MRI studies were reviewed with special attention to remnant contrast enhancement in fibroid tissue. The study design did not include any masking and the results of CEUS were accessible to the radiologists assessing the MRI examinations. The results of the MRI studies were compared with the final CEUS examination.
The study was approved by the regional ethics committee and all patients signed written informed consent.
Results
In all patients, the enlarged uterus was clearly visible by transabdominal ultrasound, and CEUS was successfully performed before and immediately after embolization. There was early contrast enhancement in fibroids prior to UAE in all cases.
Technically successful embolization, defined as bilateral embolization of uterine arteries, was achieved in all patients. Based on the results of CEUS the embolization was continued in 4 patients in spite of the fact that the angiographic end-point had already been reached. In 2 patients further embolization was carried out once and in 2 other patients twice toward near stasis in the uterine arteries. In 6 patients the procedure was ended after the first ultrasound examination (Table 1).
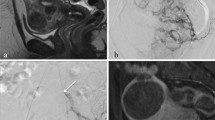
Based on a review of the series we retrospectively classified four different types of imaging outcome: type 1 denotes complete absence of contrast enhancement in fibroids (Fig. 1), type 2 indicates contrast enhancement in a segment- or a sector-like part of dominant fibroids (Fig. 2), and type 3 refers to contrast enhancement in a peripheral rim of fibroid tissue surrounding an ischemic center (Fig. 3). In cases of multiple fibroids incomplete embolization can result in complete devascularization of some fibroids while others show unchanged contrast enhancement (Fig. 4). This mosaic pattern was defined as type 4 outcome.
Type 3 outcome. A CEUS shows a contrast-enhancing rim surrounding a low-echogenic center. High-echogenic spots in the centre are artifacts from air bubbles; these could also be seen prior to intravenous administration of contrast medium. B MRI 3 months post-embolization shows an increased size of the vascularized zone
Type 4 outcome. MRI 3 months post-embolization in a patient with multiple fibroids. There is complete devascularization of fibroids in the uterine fundus (arrowhead) and preserved vascularization of fibroids in the lower part of the uterine body (arrows). At UAE, CEUS confirmed incomplete embolization due to a patent ovarian artery
In 7 of the 10 patients final CEUS suggested complete devascularization of fibroids.
One of these was patient 2, with multiple myomas (patient 2, Table 1), who previously had undergone myomectomy. Angiography showed collaterals from the right ovarian artery. CEUS immediately after UAE was technically difficult because of the multiplicity of fibroids, but the findings were interpreted as complete devascularization of all fibroids.
In patient 5 (Table 1) the end-point of UAE was adjusted twice based on the finding at CEUS. At final CEUS findings were misinterpreted as completely devascularized fibroid surrounded by myometrium. Retrospectively, there was a rim of contrast-enhancing fibroid tissue and only central devascularization of the largest fibroid (type 3 outcome, Fig. 3A).
The sixth patient in our series was referred to UAE several years after myomectomy. Because of collaterals from a small ovarian artery, we were technically unable to obtain complete embolization of all fibroids. This was correctly predicted by CEUS at the end of the procedure (type 4 outcome, Fig. 4).
Postprocedural MRI confirmed complete devascularization of all fibroids in 7 of 10 patients both on 1 day and 3 months following UAE. In patient 2, MRI on the day after UAE suggested complete embolization; however, there was poor differentiation between fibroids and myometrium. At 3 months, MRI clearly showed enhancing fibroids mainly located dorsally on the right side of the uterus (Fig. 4), but there was marked volume reduction of the uterus and the patient had complete relief of symptoms.
In patient 5, who had a fibroid with central necrosis and a vascularized outer rim at completion of UAE (Fig. 3A), MRI at 3 months follow-up showed an increased size of the viable part of the fibroid (Fig. 3B).
Discussion
Former studies have shown that the end-point of the angiographic procedure is crucial to the clinical success of UAE [1, 8]. Embolization that is too aggressive can lead to endometritis, possibly necessitating hysterectomy, and increases the risk of unintended embolization of other organs. If the embolization is incomplete and there remains partial vascularization of fibroids, the patient is at risk of having a poor clinical outcome due to regrowth of the fibroids.
Even when UAE is performed by experienced interventional radiologists, follow-up MRI examinations may show incomplete devascularization of fibroids in up to 20% of cases [1, 9, 10]. This can be due to either technical or anatomic reasons. Fluoroscopy is not perfect when it comes to detecting a low concentration of contrast medium. Establishing the correct end-point by angiography alone is technically challenging. Suggested end-points such as the “pruned-tree” appearance or “sluggish forward flow in the uterine artery”, where 2 ml of injected contrast material can be observed during five heart beats [8], leave room for subjective interpretation. In addition, there is a need for different angiographic end-points when using different embolic agents, as shown by Spies et al. and Golzarian et al. when using spherical polyvinyl-alcohol particles [2, 11].
MRI with contrast-enhanced series is considered the gold standard for evaluation of the degree of devascularization after UAE. However, it can not be performed during the procedure and does not allow immediate adjustment of the end-point of embolization.
Blood supply to the fibroids from the ovarian arteries is a well-described reason for UAE failure [12, 14]. Anastomoses of the uterine and ovarian arteries are present in up to 45% of women undergoing UAE [15], and Razavi et al. found independent blood supply from the ovarian artery directly to fibroids in 6.6% of 76 patients [16]. Previous surgery increases the incidence of significant collaterals and may have contributed to a relatively high rate of only partial devascularization in our series.
In 3 of our patients (30%) we were not able to achieve complete devascularization of all fibroids. In 2 cases this was due to ovarian collaterals.
Because of concerns regarding radiation dose, we do not routinely perform aortography for the detection of collaterals from the ovarian arteries. USCA injected intravenously will reach the uterus by whichever collateral there might be, while the X-ray contrast medium arrives only through the specific artery being catheterized. In 2 patients with collateral blood supply to the fibroids, MRI revealed incomplete devascularization after 3 months. This was correctly predicted by CEUS during the procedure in 1 patient. In the second patient, both CEUS during UAE and MRI the following day suggested complete devascularization. Due to a multiplicity of fibroids, interpretation of the early examinations was difficult in both modalities, and there was no clear benefit of early MRI as compared with CEUS.
In our series we wanted to prospectively evaluate the feasibility of CEUS during UAE and to compare CEUS with early postprocedural MRI. We did not systematically compare different angiographic end-points with the degree of remaining contrast enhancement in fibroids on CEUS, and our study does not provide a valid comparison between postprocedural CEUS and MRI. However, although the study was not masked and the number of patients was small, the correlation between postprocedural CEUS and MRI was good. The technique could be successfully performed in all patients and had an impact on the end-point of the procedure in 4 of 10 patients. This fact, however, reveals that our study design had an important bias, as we continued embolization in these 4 patients solely on the basis of the findings at CEUS. A good correlation between MRI and CEUS was assumed, but has not yet been proven for uterine fibroids.
There certainly is a learning curve in using CEUS during UAE, as demonstrated by the case where the CEUS findings were misinterpreted. This case, with an unusual type of remnant, circular contrast enhancement, initiated our classification of imaging outcome after UAE. A possible correlation between the type of imaging outcome and clinical response remains to be investigated.
In our opinion there are several interesting potential applications for the use of CEUS during UAE in the future. First, CEUS can be used by interventionists learning to perform UAE. In the early years after its introduction, UAE was performed mainly in centers experienced in arterial embolization procedures. As UAE becomes more widely available, less experienced interventionists will start performing it, and the use of CEUS during UAE might facilitate assessment of the degree of devascularization achieved. Further, CEUS could serve as a tool in research, for example in the introduction of new embolic agents. Finally, one might even propose the routine use of CEUS during UAE. We believe that CEUS can reliably detect collateral flow from the ovarian arteries and probably decreases the need for additional angiograms. Thus, both the radiation dose and the amount of contrast medium needed can be reduced. In addition, it may increase the quality of UAE by reducing the probability of partial devascularization.
Contrast-enhanced ultrasound is a feasible technique during UAE. The findings at CEUS can serve to adjust the end-point of embolization and seem to correlate well with postprocedural MRI. The technique may decrease the risk of clinical failure, the need for reinterventions, and the radiation dose. However, further studies are needed to reveal clinical benefit.
References
Pelage JP, Guaou NG, Jha RC, et al. (2004) Uterine fibroid tumors: Long-term MR imaging outcome after embolization. Radiology 230:803–809
Spies JBM, Allison SM, Flick PM, et al. (2005) Spherical polyvinyl alcohol versus tris-acryl gelatin microspheres for uterine artery embolization for leiomyomas: Results of a limited randomized comparative study. J Vasc Interv Radiol 16:1431–1437
Porter TR, Xie F (1995) Transient myocardial contrast after initial exposure to diagnostic ultrasound pressures with minute doses of intravenously injected microbubbles : Demonstration and potential mechanisms. Circulation 92:2391–2395
Rim SJ, Leong-Poi H, Lindner JR, et al. (2001) Quantification of cerebral perfusion with “real-time” contrast-enhanced ultrasound. Circulation 104:2582–2587
Wei KM, Jayaweera ARP, Firoozan SM, et al. (1998) Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97:473–483
Marret H, Tranquart F, Sauget S, et al. (2004) Contrast-enhanced sonography during uterine artery embolization for the treatment of leiomyomas. Ultrasound Obstet Gynecol 23:77–79
Pelage JP, Le Dref O, Beregi JP, et al. (2003) Limited uterine artery embolization with tris-acryl gelatin microspheres for uterine fibroids. J Vasc Interv Radiol 14:15–20
Spies JB (2003) Uterine artery embolization for fibroids: Understanding the technical causes of failure. J Vasc Interv Radiol 14:11–14
Dorenberg EJ, Novakovic Z, Smith HJ, et al. (2005) Uterine fibroid embolization can still be improved: Observations on post-procedure magnetic resonance imaging. Acta Radiol 46:547–553
Banovac F, Ascher SM, Jones DA, et al. (2002) Magnetic resonance imaging outcome after uterine artery embolization for leiomyomata with use of tris-acryl gelatin microspheres. J Vasc Interv Radiol 13:681–688
Golzarian J, Lang E, Hovsepian D, Kroncke T, et al. (2006) Higher rate of partial devascularization and clinical failure after uterine artery embolization for fibroids with spherical polyvinyl alcohol. Cardiovasc Intervent Radiol 29:1–3
Nikolic BM, Spies JBM, Abbara SM, et al. (1999) Ovarian artery supply of uterine fibroids as a cause of treatment failure after uterine artery embolization: A case report. J Vasc Interv Radiol 10:1167–1170
Razavi MK, Wolanske KA, Hwang GL, et al. (2002) Angiographic classification of ovarian artery-to-uterine artery anastomoses: Initial observations in uterine fibroid embolization. Radiology 224:707–712
Matthew M, Anthony N, Anna-Maria B (2000) Anastomoses of the ovarian and uterine arteries: A potential pitfall and cause of failure of uterine embolization. Cardiovasc Intervent Radiol 23:393–396
Kim HSM, Tsai JB, Patra AM, et al. (2006) Effects of utero-ovarian anastomoses on clinical outcomes and repeat intervention rates after uterine artery embolization. J Vasc Interv Radiol 17:783–789
Razavi MK, Hwang G, Jahed A, et al. (2003) Abdominal myomectomy versus uterine fibroid embolization in the treatment of symptomatic uterine leiomyomas. AJR Am J Roentgenol 180:1571–1575
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorenberg, E.J., Jakobsen, J.Å., Brabrand, K. et al. The Feasibility of Contrast-Enhanced Ultrasound During Uterine Artery Embolization: A Pilot Study. Cardiovasc Intervent Radiol 30, 882–887 (2007). https://doi.org/10.1007/s00270-007-9007-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-007-9007-8