Abstract
We report 2 cases of hemorrhagic complications related to use of the Angio-Seal hemostatic closure device that were successfully managed with stent-grafts. Two patients with subarachnoid hemorrhage were referred to our departments for endovascular treatment of ruptured intracranial aneurysms. The treatment was performed through a femoral access; the sheaths were removed immediately after the procedures, and the punctures sites closed by Angio-Seals. Both patients presented clinical signs of hypovolemic shock after treatment. The diagnosis of active bleeding through the puncture site was made by emergency digital subtraction angiography. The lesions were managed with stent-grafts. The use of stent-grafts proved to be efficient in the management of these life-threatening hemorrhagic complications following the use of the Angio-Seal hemostatic closure device.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Advances in cardiology, endovascular surgery, and interventional neuroradiology have required larger arterial accesses as well as the use of antiplatelet and anticoagulation therapies during and after endovascular procedures, thus increasing the risks of manual compression of the femoral punctures [1]. In the last decade, several hemostatic devices have been developed to provide rapid hemostasis for patients under strong anticoagulation and antiplatelet therapies.
The Angio-Seal hemostatic closure device (Daig-St. Jude Medical, St. Paul, MN, USA) has been widely used in our interventional neuroradiology practice and proven to be efficient, allowing prompt retrieval of the sheaths after the procedures [2]. We report 2 cases of hemorrhagic complications that occurred after the deployment of an Angio-Seal without evidence of external hemorrhage. Due to the prompt diagnosis of the active bleeding, treatment was possible by an endovascular approach with stent-grafts. To our knowledge, this is the first report of hemorrhagic complications related to a hemostatic closure device endovascularly managed with stent-grafts.
Case Reports
Case 1
A 45-year-old woman was referred to our department for endovascular treatment of a ruptured intracranial aneurysm of the right choroidal artery, responsible for a subarachnoid hemorrhage (SAH). The patient had a Glasgow score of 15 at the time of her admission to the hospital. The CT scan showed a Fisher grade 3 SAH.
With the patient under general anesthesia, the aneurysm was treated via an endovascular approach. The procedure was uneventful. A right femoral approach was performed and a 6 Fr sheath (Medikit, Tokyo, Japan) was inserted. It is important to note that this patient was obese, making the femoral puncture difficult. The standard anticoagulation protocol for ruptured aneurysms was followed: 5,000 IU of heparin was administered intravenously after the femoral puncture, followed by continuous administration of 2,000–3,000 IU/hr, to keep the activated clotting time (ACT) between 200 and 300. After the procedure the 6 Fr sheath was retrieved, following our protocol, and an Angio-Seal 6 Fr was deployed, without any sign of complication.
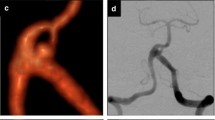
The patient was extubated after the procedure and transferred to our intensive care unit (ICU) for observation. Five days after the treatment, when she started to walk, she complained of abdominal pain and presented signs of hypovolemic shock. A CT scan was performed that showed a hyperdense collection displacing the bladder. The patient underwent DSA via the left common femoral approach (5 Fr sheath; Medikit); contralateral catheterization was performed and the angiogram showed active bleeding from the external iliac artery, revealing a puncture site higher than usual (Fig. 1).
The 5 Fr sheath was replaced by an 8 Fr sheath (Medikit), and an 8 Fr guiding catheter (Soft-tip, Boston Scientific/Target, Plymouth, MN, USA) was placed in the common iliac artery, over a 0.035-inch hydrophilic guidewire (Kayak, Boston Scientific, Miami, FL, USA). A 5.5 mm × 20 mm Symbiot self-expandable stent-graft (Boston Scientific/Scimed, Maple Grove, MN, USA) was placed to cover the rupture, over a Transend EX 0.014-inch guidewire (Boston Scientific/Target, Miami, FL, USA). After stent deployment, a Maverick 6 mm × 20 mm balloon (Boston Scientific) was advanced through the same guidewire, and inflated inside the stent. The angiographic series after deployment showed control of the bleeding, and patency of the external iliac artery (Fig. 2).
The patient’s hemodynamic status was stabilized. The pelvic hematoma was managed conservatively. Clinical follow-up was uneventful. She was kept under aspirin (250 mg daily) for 6 months and under clopidogrel (75 mg daily) for 1 month.
Case 2
A 29-year-old man was referred to our department for endovascular treatment of a ruptured aneurysm of the anterior communicating artery. The patient had a Glasgow score of 15 on arrival and the CT scan indicated a Fisher grade 2 SAH.
Endovascular treatment of the aneurysm was performed in accordance with our protocol. At the end of the treatment, a thrombus appeared at the level of the aneurysm neck and was controlled by an intra-arterial injection of 4 mg of Abciximab (Reopro, Centocor, Malvern, PA, USA), with resolution of the thrombus. A unilateral femoral approach was performed with a 6 Fr sheath, which was removed after the procedure and an Angio-Seal 6 Fr was placed, with no signs of bleeding. Other than intra-arterial administration of Abciximab, the anticoagulation protocol of this patient followed our usual protocol for ruptured aneurysms.
One hour after treatment, when the patient was already awake and in the ICU, he presented signs of hypovolemic shock associated with acute abdominal syndrome. It is important to note that there was no evidence of external bleeding from the femoral puncture. DSA was performed as an emergency procedure, via the left femoral artery, showing active bleeding from the right common femoral artery (Fig. 3). A 5 Fr sheath was used at the beginning of the procedure, but was replaced by an 8 Fr sheath which allowed the introduction of an 8 Fr guiding catheter (Soft-tip, Boston Scientific/Target) for stent deployment. A 28 mm balloon-expandable stent-graft (4–9 mm diameter range; Jostent Peripheral Stent-Graft, JOMED International, Stockholm, Sweden), manually crimped on a 6 mm × 60 mm Fox PTA balloon (JOMED International) was deployed as an emergency procedure to cover the rupture, preserving the patency of the artery (Fig. 4). After stent placement, the patient was kept under the same antiplatelet therapy which was used for the first patient.
The pelvic hematoma was treated conservatively, and clinical follow-up was uneventful. The patient’s right femoral artery was checked at the 6 month follow-up DSA for his intracranial aneurysm, and showed a patent artery without signs of stenosis.
Discussion
The development of new neurointerventional tools, such as stents, stent-grafts, bioactive coils, and liquid agents has required stronger anticoagulation and antiplatelet therapies to ensure the safety of these devices [3]. Access via the common femoral artery is still the most commonly used method in neurointerventional practice, and sheath removal is a problem under the aggressive anticoagulation protocols which are used during and, frequently, after procedures to maintain the patency of the intracranial vessels.
During the last decade, many types of hemostatic closure devices have been developed in an attempt to solve the arterial access complications that have arisen due to the development of endovascular techniques in interventional cardiology as well as in peripheral and neurointerventional radiology [4]. Although it is still not clear whether these devices are better than manual compression, their use has increased in the daily practice of endovascular specialties [5, 6], and they are more comfortable for both the physician and the patient due to the possibility of early mobilization [7].
In our practice, we have chosen the Angio-Seal (Daig-St. Jude Medical), a hemostatic device with collagen plugs, which is currently marketed as an STS platform. This version is the second available in the market. A comparative study evaluating the comparative risks of the old (Millennium) and new (STS) platforms showed that the STS platform is easier to deploy, with no significant differences in complication rate [8]. We perform nearly 500 interventional neuroradiology procedures a year and use of the Angio-Seal has been routine in our department since the beginning of 1997.
Major complications involving the Angio-Seal were observed in 0.8–3.6% of patients [4]. The most frequent complication is arterial occlusion (9), followed by embolic events [10], late stenosis, and infections [11]. Hemorrhagic complications have also been described, requiring either surgical or conservative management [10]. Here we have described 2 cases of hemorrhagic complications related to the Angio-Seal hemostatic closure device on an STS platform. The patients presented clinical signs of hypovolemic shock without external bleeding. The complications were managed by an endovascular approach with stent-grafts, which stopped the hemorrhaging and promoted good clinical outcomes.
It is important to point out that one of the patients (case 1) was obese; also the puncture, which was done in a high position (external iliac artery, above the inguinal ligament), was difficult to perform. A study that evaluated the risks of the Angio-Seal in overweight patients did not show higher complication rates for these patients compared with normal subjects [12]. Nevertheless, the difficulty in performing the puncture in our patient and the fact that it was performed in a higher position may be related to this complication. The puncture and the Angio-Seal placement for this patient were performed by a senior interventional neuroradiologist, but the femoral angiogram before arterial closure (manufacturer’s guidelines) was not performed, and it should have contraindicated placement of the device. The placement of the Angio-Seal above the inguinal ligament may result in a retroperitoneal hematoma. Nevertheless, manual compression would also have carried a major risk of bleeding at this site. Thus, for this patient both options for hemostasis could have carried a risk for a hemorrhagic complication. It is interesting that this patient presented the hemorrhage on the fifth day after the procedure, when she started to ambulate. We suppose that the hematoma, which may have been formed around the vessel immediately after the procedure, may have been mobilized by the ambulation, leading to rebleeding and its symptoms.
The second patient (case 2) presented a small thrombus at the level of the aneurysmal neck that required intra-arterial perfusion of Abciximab. It has been shown that the administration of Abciximab does not increase the rate of complications related to the use of the Angio-Seal [13]; also, the intra-arerial dose was much lower than that used in interventional cardiology practice. A balloon-expandable stent-graft was used instead of a self-expandable one for this patient. We know that, due to the location of the bleeding in a flexure point of the common femoral artery, the stent used was not the most indicated, but our department is located in a head and neck hospital, with no vascular surgery department, and the Jostent was the only device available. This patient was also controlled with an angiogram 6 months after the treatment, which showed no signs of stent crush or stenosis. Long-term follow-up will be important to evaluate the patency of the femoral artery.
Since the bleeding in our patients was not external, the diagnoses were confirmed by angiograms which showed active bleeding at the rupture site, allowing endovascular management of the lesions with stent-grafts during the same procedure and sparing the patient from open surgery.
Stent-grafts have been successfully used for endovascular repair of arterial ruptures [14–17], but to our knowledge their use in the management of active bleeding related to a hemostatic closure device, resulting in hypovolemic shock, has never been described.
In summary, we describe a serious hemorrhagic complication following use of the Angio-Seal hemostatic closure device. The arterial ruptures were successfully managed by an endovascular approach and stent-graft deployment, which proved to be an alternative to open surgery. Covered stents should be available in any department where endovascular procedures are performed, in case of an emergency such as an arterial rupture. This can avoid surgery and might save a patient’s life.
References
Bogart DB, Bogart MA, Miller JT, Farrar MW, Barr WK, Montgomery MA (1995) Femoral artery catheterization complications: A study of 503 consecutive patients. Cathet Cardiovasc Diagn 34:8–13
Kapadia SR, Raymond R, Knopf W, et al. (2001) The 6 Fr Angio-Seal arterial closure device: Results from a multimember prospective registry. Am J Cardiol 87:789–791
Pelz D, Andersson T, Lylyk P, Negoro M, Soderman M (2005) Stroke review: Advances in interventional neuroradiology 2004. Stroke 36:211–214
Hoffer EK, Bloch RD (2003) Percutaneous arterial closure devices. J Vasc Interv Radiol 14:865–885
Amin FR, Yousufuddin M, Stables R, et al. (2000) Femoral haemostasis after transcatheter therapeutic intervention: A prospective randomised study of the Angio-Seal device vs the Femostop device. Int J Cardiol 76:235–240
Applegate RJ, Grabarczyk MA, Little WC, et al. (2002) Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol 40:78–83
Yee KM, Lazzam C, Richards J, Ross J, Seidelin PH (2004) Same-day discharge after coronary stenting: A feasibility study using a hemostatic femoral puncture closure device. J Interv Cardiol 17:315–320
Lasic Z, Mehran R, Dangas G, et al. (2004) Comparison of safety and efficacy between first and second generation of Angio-Seal closure devices in interventional patients. J Invas Cardiol 16:356–358
Eidt JF, Habibipour S, Saucedo JF, et al. (1999) Surgical complications from hemostatic puncture closure devices. Am J Surg 178:511–516
Kirchhof C, Schickel S, Schmidt-Lucke C, Schmidt-Lucke JA (2002) Local vascular complications after use of the hemostatic puncture closure device Angio-Seal. Vasa 31:101–106
Eggebrecht H, Haude M, Baumgart D, Erbel R (2000) Infectious complications related to the use of the Angio-Seal hemostatic puncture closure device. Catheter Cardiovasc Interv 49:352–353
Wong P, Harding S, Walters D, Hull ML, Jang IK (2001) Vascular complications after hemostatic puncture closure device (Angio-Seal) are not higher in overweight patients. J Invas Cardiol 13:623–625
Exaire JE, Dauerman HL, Topol EJ, et al. (2004) Triple antiplatelet therapy does not increase femoral access bleeding with vascular closure devices. Am Heart J 147:31–34
Thalhammer C, Kirchherr AS, Uhlich F, Waigand J, Gross CM (2000) Postcatheterization pseudoaneurysms and arteriovenous fistulas: Repair with percutaneous implantation of endovascular covered stents. Radiology 214:127–131
Criado E, Marston WA, Ligush J, Mauro MA, Keagy BA (1997) Endovascular repair of peripheral aneurysms, pseudoaneurysms, and arteriovenous fistulas. Ann Vasc Surg 11:256–263
Chan RP, Common AA (2004) Stent-graft repair of femoral pseudoaneurysm/AV fistula using a retrograde popliteal approach. Cardiovasc Intervent Radiol 27:516–519
Onal B, Kosar S, Gumus T, Ilgit ET, Akpek S (2004) Postcatheterization femoral arteriovenous fistulas: Endovascular treatment with stent-grafts. Cardiovasc Intervent Radiol 27:453–458
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giansante Abud, D., Mounayer, C., Saint-Maurice, J.P. et al. Stent-Grafts in the Management of Hemorrhagic Complications Related to Hemostatic Closure Devices: Report of Two Cases. Cardiovasc Intervent Radiol 30, 104–107 (2007). https://doi.org/10.1007/s00270-005-0321-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-005-0321-8