Abstract
Purpose
To evaluate the feasibility and effectiveness of feeding tube insertion and enteral feeding for the treatment of postoperative gastrointestinal anastomotic obstruction and leakage.
Materials and Methods
From June 1999 to June 2002, thirty-four cases of postoperative gastrointestinal anastomotic obstruction and leakage after surgery for gastric carcinoma were treated by insertion of a feeding tube under fluoroscopic guidance. Twenty-one patients were male and 13 were female. The patients’ ages ranged from 39 to 74 years (mean age: 61 years). All the patients experienced vomiting, and 15 patients had anastomotic site or duodenal stump leakage. We evaluated the feasibility of feeding tube insertion for enteral feeding to improve the obstruction and facilitate leakage site closure, and the patients’ nutritional benefit was also evaluated by checking the serum albumin level between pre- and post-enteral feeding via the feeding tube.
Results
Thirty-two patients (94%) were successfully managed by feeding tube insertion, but the remaining two were not managed, and this was due to severe angulations at the anastomotic site. The procedure times for feeding tube insertion ranged from 15 to 60 minutes (mean time: 45 minutes). Twenty-eight patients experienced symptomatic relief of gastrointestinal obstruction, and they were able to resume a normal regular diet after feeding tube removal. Three patients underwent stent insertion due to recurrent symptoms, and one patient underwent jejunostomy feeding due to the presence of a persistent leakage site. Eleven patients achieved leakage site closure after enteral feeding via a feeding tube. The serum albumin level was significant, increased from pre-enteral feeding (2.65 ± 0.37 g/dL) to the post-enteral feeding (3.64 ± 0.58 g/dL) via the feeding tube (p < 0.001). The duration of follow-up ranged from one to 53 months (mean: 23 months).
Conclusion
The insertion of a feeding tube for enteral feeding under fluoroscopic guidance is safe, and it provides effective relief from gastrointestinal anastomotic site obstruction and leakage after gastric surgery. Moreover, our findings indicate that feeding tube insertion for enteral feeding may be used as the primary procedure to treat postoperative anastomotic obstruction and leakage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although the amounts of postoperative anastomotic leakage can be rather slight, this leakage is a major source of mortality and morbidity [1]. Surgical re-exploration and repair is the principal treatment for most cases of postoperative anastomotic leakage and obstruction [2]. Postoperative anastomotic leakage and obstruction have typically been managed by prolonged nasogastric suction, fluid replacement, broad-spectrum antibiotics, and parenteral alimentation [1, 2].
The possible causes of anastomotic leakage are arterial insufficiency, venous insufficiency, tension, technical errors, gastric distension, infection, and extrinsic compression [3]. The technical errors that have been associated with anastomotic leakage include tying the surgical sutures too tightly and/or placing an excessive number of sutures, which causes strangulation of the anastomotic tissue. Roy-Choudhury et al. [4] and Kwak et al. [5] reported on the successful treatment of gastrojejunal or gastroesophageal anastomotic leakage with using a covered metallic stent. However, there are no reports available regarding the placement of a feeding tube for the relief of gastrointestinal anastomotic obstruction and leakage.
In this study, we determined the feasibility and effectiveness of using feeding tube insertion for enteral feeding in the treatment of postoperative gastrointestinal anastomotic obstruction and leakage. The effect on nutritional status was evaluated by serum albumin levels.
Materials and Methods
Thirty-four patients suffering with postoperative gastrointestinal anastomotic obstruction and leakage underwent feeding tube insertion for enteral feeding under fluoroscopic guidance from June 1999 to June 2002. The 34 patients underwent surgery for gastric carcinoma. Twenty-one patients were male and 13 were female, and their ages ranged from 39 to 74 years (mean age: 61 years). Seven of the patients were TNM stage I gastric carcinoma patients, 4 were TNM stage II, 9 were TNM stage III, and 14 were TNM stage IV. The present study was approved by the Institutional Review Board of our university and the Ethics Committee of the Institute for Medical Science. The procedure was explained to all of the patients in detail, and a written consent was obtained from all the patients prior to the procedure.
The study inclusion criteria were (1) the presence of an anatomical stenosis or obstruction on the radiographic studies, (2) patient symptoms such as vomiting, or (3) a gastrointestinal series with gastrografin that showed anastomotic site leakage and duodenal stump leakage. The exclusion criteria were (1) a lack of patient cooperation during the procedure and (2) the presence of a severe septic or disseminated intracoagulopathic state following surgery.
The patients were divided into two groups: one group contained those patients with acute obstruction or leakages within 31 days postoperatively, and the other group contained those patients with chronic obstruction or leakages at more than 31 days postoperatively. Twenty-six of the 34 patients experienced acute obstruction or leakages, and 8 patients had chronic obstruction or leakages. All of the patients experienced vomiting, and 15 had anastomotic site leakage or duodenal stump leakage. An upper gastrointestinal series with gastrografin showed anastomotic obstruction in these 15 patients; anastomotic site leakage was found in 1 patient and duodenal stump leakage was found in 14 patients.
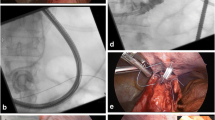
The upper gastrointestinal series (UGIS) with gastrografin that was taken before feeding tube insertion revealed anastomotic site obstructions and leakages at the anastomoses and duodenal stumps (Fig. 1a). When a leakage was found to be connected with the pleura or lung parenchyma, UGIS with barium was performed because gastrografin is toxic to the lung parenchyma and pleura. Pharynx topical anesthesia was routinely performed using lidocaine gel before feeding tube insertion. Neither sedatives nor general anesthetic were used. A 0.035-in. angled exchange guidewire (Radiofocus wire; Terumo, Tokyo, Japan) was inserted through the nose under fluoroscopic guidance (Angiostar; Siemens, Erlangen, Germany) into the remaining portion of the stomach or esophagus, and a headhunter catheter (Cook, Bloomington, IN) or a coronary guide catheter (Cordis, Miami, FL) was then inserted via the guidewire. The gastrografin was injected through the catheter to visualize the exact site of the anastomotic obstruction and leakage of the anastomosis or duodenal stump. The catheter was then advanced across the anastomotic site into the jejunum (Fig. 1b). Gastrografin was reinjected through the catheter to locate the feeding tube insertion site. An Amplatz superstiff exchange guidewire (Medi-Tech/Boston Scientific, Watertown, MA) was then inserted via the catheter into the distal portion of the jejunum (Fig. 1c), and a feeding tube (Flexiflo®; 12F, 114 cm; Abbott, Zwolle, The Netherlands) was inserted into the jejunum using the guidewire (Figs. 1d and 2). After the procedure, UGIS with gastrografin was performed to confirm the location of the feeding tube.
The feeding tube insertion procedure. (A) UGIS showing the duodenal stump leakage (arrow). (B) A Terumo exchange guidewire was inserted through the anastomotic site into the jejunum of the efferent loop. (C) An Amplatz exchange guidewire was inserted through the efferent loop away from the anastomotic site into the jejunum. (D) A feeding tube located in the jejunum of the efferent loop through the anastomotic site.
All of the patients underwent hyperalimentation via a feeding tube. When the follow-up UGIS showed good patency of the anastomotic site and closure of the leakage sites, the feeding tubes were removed.
Immediate technical success was defined as successful insertion of the feeding tube without any problems. Clinical success was defined as complete improvement of the anastomotic site obstruction and closure of the leakage site, as was confirmed by the patients’ clinical food intake and follow-up UGIS. The patients’ nutritional benefit was evaluated by the serum albumin level change between the pre- and post-enteral feeding via the feeding tube. The differences in the means were evaluated with the use of t-tests.
Results
Immediate technical success was achieved in 94% of the patients (32/34 patients) (Table 1). All patients were initially recommended for an endoscopic procedure. However, not all of the patients had successful feeding tube insertion via endoscopic guidance by an endoscopist. In two patients, the feeding tube insertion was unsuccessful because of severe angulation of the anastomotic site. One of these two patients underwent endoscopic insertion of the guidewire, but the procedure failed because the endoscope did not advance into the anastomotic site. The acute group (<31 postoperative day) showed a high incidence of leakage (14/26 = 54%), but the chronic group (>31 postoperative day) showed a low incidence of leakage (1/8 = 13%).
The procedure times for feeding tube insertion ranged from 15 to 60 min. (mean time: 45 min.). The procedure time was dependent on the detection of the anastomotic site and the passage of the catheter over the guidewire. In cases of postoperative Billroth I, the anastomotic site was easily located, and guidewire and catheter passage was achieved without great difficulty, whereas in cases of postoperative Billroth II, although the guidewire and catheter were easily inserted into the afferent loop, finding the efferent anastomotic site and the passage of these wires into the efferent loop were difficult due to the severe angulation. The feeding tubes were retained in position for 2–50 days (mean time: 11 days).
Twenty-eight patients (28/32 = 87.5%) experienced symptomatic relief of their gastrointestinal obstruction and they were able to eat a normal diet. Eleven patients with leakage at the anastomotic site or duodenal stump (11/15 = 73%) showed leakage site closure (Fig. 3). The cases of afferent loop distention and duodenal stump leakage showed efferent loop obstruction. These patients had not taken food and, therefore, the gastric juice secretion was reduced. In these cases, the duodenal stump leakage stopped after the hyperalimentation through a feeding tube that was placed in the efferent jejunal loop. One patient underwent feeding jejunostomy due to a persistent anastomotic leakage (Fig. 4). Three patients underwent stent insertion because of their recurrent symptoms of gastrointestinal obstruction during the follow-up period (at 2, 4, and 28 months, respectively) after feeding tube removal. The follow-up duration ranged from 1 to 53 months (mean: 23 months).
A 70-year-male who underwent radical distal gastrectomy. (A) UGIS showing an anastomotic site obstruction (white arrow) and anastomotic site leakage (black arrow). (B) A feeding tube was inserted through the anastomotic site into the proximal jejunum. (C) Three-year follow-up UGIS showing good patency of the anastomotic site and no evidence of anastomotic site leakage.
A 59-year-old male who underwent palliative subtotal gastrectomy. (A) A feeding tube was inserted into the jejunum of the efferent loop and the left pleural space was drained using a percutaneous catheter. (B) The 1-week follow-up UGIS showed a persistent, large hole at the anastomotic site (arrow), which allowed barium leakage into the pleural spaces. (C) The 2-week follow-up UGIS showed persistent barium leakage through the anastomotic site hole (arrow) into the pleural spaces.
The patient’s nutritional benefit was shown by the increasing serum albumin level after enteral feeding via the feeding tube. The mean serum albumin level was 2.65 ± 0.37 g/dL (1.90–3.70 g/dL) in the pre-enteral feeding tube period and 3.64 ± 0.58 g/dL (2.50–4.80 g/dL) in post-enteral feeding via the feeding tube. The differences in the mean were significantly increased between pre- and post-enteral feeding via the feeding tube (p < 0.001).
Discussion
Anastomotic leakage after surgery in gastric cancer patients is a major source of mortality and morbidity [1, 6]. Although several methods such as surgical re-exploration and repair and/or more conservative approaches like parenteral nutrition or broad-spectrum antibiotics have been used, no consensus has been reached on the best methods of treatment, and the rate of treatment failure (30%) remains high for carcinoma [7].
Shchepotin et al. [8] reported a major complication rate following gastrectomy of 5.7% (40/700 patients). Of these patients, anastomotic leakage and incompetence of the sutures were held responsible in 11 patients (27.5%), intra-abdominal abscess was the problem in 8 patients (20%), and pancreatic necrosis was the problem in 7 patients (17.5%). Anastomotic leakage was reported to develop on postoperative day 3.2 ± 1.2 and this manifested as peritonitis and the appearance of an exudate from the surgical drains or between the cutaneous sutures [8]. In our cases, 15/34 patients (44%) showed anastomotic or duodenal stump leakage at 6–68 days (mean: 22 days) after surgery. The high rate of complications in our cases might have been due to the delayed detection of leakage because we have usually favored allowing spontaneous healing, and so we waited until the patients complained.
The causes of anastomotic site obstruction after gastrectomy are immediate postoperative mucosal edema, benign fibrotic stricture, and recurrent cancer [4, 5]. In our study, 19 (3.4% = 19/566 patients) patients had anastomotic site obstruction, and they initially received gastric decompression through a Levine tube that was surgically inserted. Anastomotic site obstruction in these patients was demonstrated by radiographic study after removal of the Levine tube. Food intake can irritate the mucosa at the anastomotic site. However, hyperalimentation through a 12F feeding tube might reduce gastric juice secretion, and enteric feeding might contribute to correcting the general nutrition from the postsurgical status, and so this facilitates postoperative anastomotic site healing. The anastomotic site obstruction improved within 10 days of feeding tube insertion with hyperalimentation. In our opinion, the major cause of anastomotic site obstruction is persistent postoperative mucosal edema. In one case, a metallic stent was administered 2 months after feeding tube insertion because of tumor recurrence at the anastomotic site.
The cause of recurrent duodenal stump leakage or anastomotic leakage despite surgical management might be due to the presence of pressure between the residual stomach and the jejunum that is caused by luminal narrowing of the efferent limb due to its acute angulation. Moreover, the failure of medical treatment after leakage recurrence is probably caused by the continuous direct contact of the leakage site with the food fluid stream and the wall tension that is caused by the different luminal diameters of the residual stomach and the jejunum [9]. The retention of gastric juice in the afferent loop might increase pressure in the residual stomach and in the afferent loop and thus cause duodenal stump leakage. The peroral intake of food stimulates gastric juice secretion, which irritates the gastric mucosa; thus, the use of a feeding tube might reduce mucosal irritation. Treatment by hyperalimentation through a feeding tube tends to relieve the postoperative mucosal edema at the anastomotic site. The pressure gradient and the wall tension that are caused by the different luminal diameters between the residual stomach and the jejunum are reduced by the lack of food oral intake and, thus, the anastomotic obstruction is relieved; as a result, the duodenal stump leakage is stopped. For the three patients who experienced obstructive symptoms during the follow-up period, a gastric stent was inserted at the anastomotic site obstruction, and the obstructive symptoms were relieved.
Alivizatos et al. [10] have reported that octreotide rapidly reduces the fistula output and thereby accelerates the healing process. Spontaneous closure was achieved in 13/21 patients in the octreotide group (mean closure time: 15.3 days; range: 6–35) and in 12/17 patients who were treated only by total parenteral alimentation (mean closure time: 13.9 days; range: 7–25); however, this difference is not significant (p = 0.5). Octreotide has two different mechanisms; it inhibits gastrointestinal, biliary, and pancreatic secretions, and it relaxes the bowel muscle layer and allows fluid to accumulate within the bowel lumen [10]. The enteric feeding might contribute to the general nutritional correction from the postsurgical status, and this might contribute to the closure of anastomotic leakage [1, 2]. However, total parenteral nutrition is expensive and it has a high rate of complications. In general, the Levine tube drainage that is often placed during the postoperative period is not feeding tube; rather it was used for decompressing the remnant stomach and relieving the gastric secretion. The enteral feeding allows for a high serum albumin level compared to total parenteral nutrition [2]. In our study, the patient nutrition benefit showed a significantly increased serum albumin level after enteral feeding (p < 0.001).
The advantages of enteral over parenteral feeding and the administration of solutions into the small bowel rather than into the stomach to prevent reflux and aspiration have increased the demand for enteral feeding tube placement [11]. Ott et al. [11] have reported a success rate for fluoroscopic placement of 90%, with a tube placed into the jejunum in 53% of patients and placed into the duodenum in 47% of the patients. In their study, the fluoroscopic times and room times for the successful fluoroscopic placements were 8.6 ± 5.6 min (mean ± SD) and 21.7 ± 8.4 min, respectively, and for the 10 unsuccessful placements, the fluoroscopic times and total room times were 16.2 ± 5.4 min (mean ± SD) and 45.6 ± 18.4 min, respectively. In the present study, all of patients had undergone surgery; thus, the anatomic structures of the upper gastrointestine tract had been modified by surgery. The tubes were placed fluoroscopically in the jejunum in 31 patients, and the mean procedure time was 45 min.
Moreover, the endoscopic and fluoroscopic placement of postpyloric feeding tubes can be performed safely and accurately at the bedside for critically ill patients [12]. Fluoroscopic placement requires less additional sedation, whereas endoscopic placement allows direct visualization of the gastric and duodenal anatomy, and it can be performed without additional X-ray support [12]. In our study, fluoroscopic guidance using a catheter and guidewire allowed for the anastomotic sites to be found easily and there were unhindered passages of the catheter into the jejunum and duodenum because the catheter has a considerably smaller diameter than the endoscopy instrument.
In conclusion, feeding tube insertion under fluoroscopic guidance was found to provide safe and effective relief of the gastrointestinal anastomotic site obstruction and leakage that was observed after gastric surgery. We suggest that feeding tube insertion should be considered as an alternative therapeutic option for patients having an intractable postoperative anastomotic site and duodenal stump leakage after gastrojejunostomy or gastroduodenostomy.
References
Sand J, Luostarinen M, Matikaninen M (1997) Eneteral or parenteral feeding after total gastrectomy: prospective randomised pilot study. Eur J Surg 163:761–766
Papapietro K, Diaz E, Csendes A, et al. (2002) Early enteral nutrition in cancer patients subjected to a total gastrectomy. Rev Med Child 130:1125–1130
Urschel JD, (1995) Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 169:634–640
Roy-Choudhury SH, Nicholson AA, Wedgwood KR, et al. (2001) Symptomatic malignant gastroesophageal anastomotic leak: management with covered metallic esophageal stents. Am J Roentgenol 176:161–165
Kwak HS, Lee JM, Jin GY, et al. (2003) Treatment of gastrojejunal anastomotic leak with a covered metallic stent. Hepatogastroenterology 50:62–64
Salo JA, Saario I, Kivilaakso EO, et al. (1988) Near-total gastrectomy for gastric cancer. Am J Surg 155:486–489
Tarazi R, Coutsoftides T, Steiger E, et al. (1983) Gastric and duodenal cutaneous fistulas. World J Surg 7:463–473
Shchepotin IB, Evans SRT, Chorny VA, et al. (1996) Postoperative complications requiring relaparotomies after 700 gastrectomies performed for gastric cancer. Am J Surg 171:270–273
Heindel W, Gossmann A, Fischbach R, et al. (1996) Treatment of a ruptured anastomotic esophageal stricture following bougienage with a Dacron-covered nitinol stent. Cardiovasc Intervent Radiol 19:431–434
Alivizatos V, Felekis D, Zorbalas A (2002) Evaluation of the effectiveness of octreotide in the conservative treatment of postoperative enterocutaneous fistulas. Hepatogastroenterology 49:1010–1012
Ott DJ, Mattox HE, Gelfand DW, et al. (1991) Enteral feeding tubes: placement by using fluoroscopy and endoscopy. Am J Roentgenol 157:769–771
Foote JA, Kemmeter PR, Prichard PA, et al. (2004) A randomized trial of endoscopic and fluoroscopic placement of postpyloric feeding tube in critically ill patients. J Parenter Enteral Nutr 28:154–157
Acknowledgments
The author thanks Bonnie Hami, MA, of the Department of Radiology, University Hospitals of Cleveland for her editorial assistance in the preparation of this article and Kevin Condren of the Harrisco Language Research Institute for his editorial assistance in the revision of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, YM., Kim, CY., Yang, DH. et al. Fluoroscopically Guided Feeding Tube Insertion for Relief of Postoperative Gastrointestinal Anastomotic Obstruction and Leakage. Cardiovasc Intervent Radiol 29, 395–400 (2006). https://doi.org/10.1007/s00270-005-0095-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-005-0095-z