Abstract
The purpose of this study was to elucidate the role of the superior thyroid artery in intra-arterial infusion chemotherapy for laryngeal and hypopharyngeal cancers. Thirty-nine patients with laryngeal cancer and 29 patients with hypopharyngeal cancer underwent intra-arterial infusion chemotherapy. We performed a retrospective analysis of the feeding arteries confirmed by computed tomography during selective arteriography and compared the results with the extent of the tumors. In 14 of 39 laryngeal and 15 of 29 hypopharyngeal cancers, the tumor did not cross the midline (group 1). In the remaining 25 and 14 cancers, respectively, the tumor crossed the midline or located in the center (group 2). For 13 of 14 laryngeal and 7 of 15 hypopharyngeal cancers in group 1 and for 6 of 25 laryngeal cancers in group 2, the entire tumor was contrast enhanced by the ipsilateral superior thyroid and/or superior laryngeal artery. For 12 of 25 laryngeal and 1 of 14 hypopharyngeal cancers in group 2, the entire tumor was contrast enhanced by the bilateral superior thyroid artery. For the other patients, infusion via the other arterial branches such as the inferior thyroid and the lingual arteries were needed to achieve contrast enhancement of the entire tumor. Superselective intra-arterial chemotherapy for laryngeal cancer from the superior thyroid artery is appropriate, whereas that for hypopharyngeal cancer is less sufficient. To accomplish contrast enhancement of the entire tumor, additional intra-arterial infusion from other arteries such as the inferior thyroid artery is often necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Superselective intra-arterial infusion chemotherapy is sometimes attempted on patients with head and neck cancer to obtain a greater therapeutic effect, and regional intra-arterial chemotherapy has the potential advantage of delivering a higher concentration of chemotherapeutic agent to the tumor site and reducing systemic side effects [1–3].
It is essential to identify the feeding artery of a tumor for superselective intra-arterial infusion chemotherapy. Previously, digital subtraction angiography (DSA) and/or infusion of a dye agent such as indigocarmine were used to confirm the feeding artery [4]. Computed tomography (CT) during selective arteriography (CTSA) for intra-arterial infusion chemotherapy has several advantages. It allows confirmation of drug distribution even in the deep cervical region and it is easily repeated using the CT with DSA system if the selected arterial branches are found to be inappropriate. Identification and confirmation of multiple feeding arteries and the appropriate distribution of a chemotherapeutic agent are also possible. Although a CT with DSA system is useful, it has been installed in a limited number of institutions so far. The purpose of this study was to clarify the role of the superior thyroid artery in superselective intra-arterial chemotherapy for laryngeal and hypopharyngeal cancers.
Materials and Methods
From February 1999 to May 2004, 39 patients with laryngeal cancer (17 patients with glottic cancer and 22 patients with supraglottic cancer) and 29 patients with hypopharyngeal cancer underwent angiography for intra-arterial infusion chemotherapy. Four patients with recurrence were included in this study: two patients with recurrent glottic cancer after radiotherapy, one patient with recurrent supraglottic cancer, and one patient with recurrent hypopharyngeal cancer after systemic chemotherapy with concurrent radiotherapy. We performed a retrospective review of the angiographic records and clinical information. All patients gave informed consent before angiography and superselective intra-arterial infusion chemotherapy.
Angiography
Angiography was performed via the femoral artery under local anesthesia by the Seldinger technique. Systemic heparinization was accomplished with intravenous administration of 3000 IU of heparin, and superselective catheterization was performed with a coaxial catheter system using a 5F guiding catheter and a microcatheter. The guiding catheter was placed in the common carotid artery or the subclavian artery. The tip of the microcatheter was curved beforehand using steam to facilitate catheterization, and after it was inserted into the target artery supplying the tumor, DSA and CTSA were performed using CT with a DSA system (XACTIVE IVR/CT System; Toshiba Medical, Tokyo, Japan), which is a DSA system equipped with a CT scanner (Xvision/SP scanner; Toshiba Medical). For CTSA, iodinate contrast medium (Hexabrix 320, ioxaglic acid; Tanabe Seiyaku Co., Tokyo, Japan) diluted with the same volume of saline was manually injected at 0.5 mL/sec. After confirming the feeding arteries of the tumor, chemotherapeutic agents were administered. If the tumor had a single feeding artery, 100 mg of cisplatin (Briplatin; Bristol Pharmaceuticals K.K., Tokyo, Japan) was injected at 10 mL/min by a power injector. If the tumor had multiple feeding arteries, 30–100 mg of cisplatin was injected into each branch according to the bulk of the enhancement within the tumor on CTSA. In total, 100–150 mg of cisplatin was administered. For early cases of 10 laryngeal and 7 hypopharyngeal cancers, a total of 10–30 mg of pirarubicin hydrochloride (Therarubicin; Meiji Seika Kaisha, Tokyo, Japan) was additionally injected into the feeding arteries by hand. Concurrent radiotherapy (60–72 Gy/30–36 fraction) was also performed on all but four patients with recurrence.
Image Analysis
The dominant subsite of each tumor and its extent were determined by a combination of endoscopic findings, CT and magnietic resonance imaging (MRI). DSA and CTSA of the ipsilateral or bilateral superior thyroid and/or the superior laryngeal artery were performed in all patients except one patient who had undergone ligation of the superior thyroid artery. We retrospectively observed the DSA and CTSA, and identification of the arterial branches was based on the typical course as seen by DSA [5, 6].
We examined the presence of the contrast enhancement of the entire tumor on CTSA and the feeding arteries of the tumor and compared it with the dominant subsite of the tumor, the extent of the tumor, and the presence of the tumor growth across the midline.
Results
Bilateral carotid angiography was performed on 18 patients with laryngeal cancer and 10 patients with hypopharyngeal cancer, and unilateral carotid angiography was performed on the remaining 21 and 19 patients, respectively. Overall, 53 right and 43 left carotid angiographies were performed. Forty-four superior thyroid arteries were found to have arisen from the external carotid artery: 42 from the level of the carotid bifurcation and 9 from the common carotid artery. The superior thyroid artery could not be identified and the superior laryngeal artery arose from the lingual artery in a patient who had undergone ligation of the superior thyroid artery. The superior laryngeal artery was occluded in another patient. Six superior laryngeal arteries (in 5 patients) directly arose from the external carotid artery, and the remaining 89 superior laryngeal arteries arose from the superior thyroid artery. For 13 of 17 glottic cancers, 19 of 22 supraglottic cancers, and 22 of 29 hypopharyngeal cancers, tumoral blush was seen on carotid angiography.
Laryngeal Cancer
The results for the laryngeal cancer patients are summarized in Table 1. The tumor did not cross the midline in 8 of 17 glottic cancers and 6 of 22 supraglottic cancers (group 1). The tumor crossed the midline in 8 glottic cancers and 11 supraglottic cancers, and the tumor was located in the center in the remaining 1 and 5 cancers, respectively (group 2).
For 13 of 14 laryngeal cancer patients in group 1, the entire tumor was contrast enhanced by the ipsilateral superior thyroid or superior laryngeal artery (Figs. 1 and 2). For another glottic cancer patient in group 1, the ipsilateral superior laryngeal artery was occluded, and the entire tumor was enhanced by the contralateral superior laryngeal artery that directly arose from the external carotid artery.
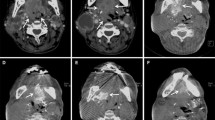
A 65 year-old man with supraglottic cancer. Axial T1-weighted MR imaging (SE, TR/TE 500/9). (A) A supraglottic tumor (arrows) in the epiglottis. Right carotid arteriography (B) and selective right superior thyroid arteriography (C). The superior thyroid artery (arrow) is arising at the level of the carotid bifurcation. The tumor is enhanced on CT during selective right superior thyroid arteriography (D).
A 49 year-old man with glottic cancer involving the vocal cord and the ventricle. (A) Contrast-enhanced CT shows the tumor in the vocal cord. (B) Right carotid arteriography shows the superior thyroid artery (arrowhead) and the superior laryngeal artery (arrow) arising directly from the external carotid artery. (C) A microcatheter is inserted into the superior laryngeal artery. (D) The tumor in the vocal cord is enhanced on CT during selective superior laryngeal arteriography.
For 6 of 25 laryngeal cancer patients in group 2, the entire tumor was contrast enhanced by the ipsilateral superior thyroid or superior laryngeal artery. For 12 of 25 patients in group 2, the entire tumor was contrast enhanced by the bilateral superior thyroid arteries. For two patients in group 2, the entire tumor was enhanced by the ipsilateral inferior thyroid artery in addition to the bilateral superior thyroid arteries. For one patient, the entire tumor was enhanced by the ipsilateral lingual artery in addition to the bilateral superior thyroid arteries. For the remaining four patients, contrast enhancement of the entire tumor was not achieved.
Hypopharyngeal Cancer
The results for the hypopharyngeal cancer patients are summarized in Table 2. The tumor did not cross the midline in 15 of 29 hypopharyngeal cancers (group 1). The tumor crossed the midline in 10 hypopharyngeal cancers, and it was located in the center in the remaining 4 (group 2).
For 7 of 15 hypopharyngeal cancer patients in group 1, the entire tumor was contrast enhanced by the ipsilateral superior thyroid artery and superior laryngeal artery. For two patients in group 1, the entire tumor was enhanced by the ipsilateral inferior thyroid artery in addition to the ipsilateral superior thyroid artery.
For three hypopharyngeal cancers in group 2, the entire tumor was enhanced by the ipsilateral inferior thyroid artery in addition to the bilateral superior thyroid arteries. The entire tumor was enhanced by the bilateral superior thyroid arteries, by the ipsilateral superior and inferior thyroid arteries (Fig. 3), by the bilateral superior and inferior thyroid arteries, and by the ipsilateral superior and contralateral inferior thyroid arteries for each hypopharyngeal cancer in group 2. The other three hypopharyngeal cancers in group 2 were enhanced by the ipsilateral lingual artery in addition to the superior and/or inferior thyroid arteries (Fig. 4).
A 79 year-old male with hypopharyngeal cancer. (A) Axial T2-weighted MR imaging (FSE, TR/TE 4000/99) shows the tumor in the piriform sinus. Selective right superior (B) and inferior thyroid arteriography (C) are shown. (D) Most of the tumor is enhanced on CT during selective right superior thyroid arteriography. (E) The posterior part of the tumor is enhanced on CT during selective inferior thyroid arteriography.
A 52 year-old man with hypopharyngeal cancer. Axial contrast enhanced MR imaging (SPGR, TR/TE/FA 170/1.5/90). (A) The tumor involving the posterior wall of the hypopharynx and the left piriform sinus. (B) Left carotid arteriography shows the superior thyroid and lingual arteries arising from the external carotid artery. Selective left superior thyroid (C) and lingual (D) arteriography are shown. (E) CT during selective left superior thyroid arteriography shows contrast enhancement of the lateral part of the tumor and (F) CT during selective left lingual arteriography shows that of the mesial part of the tumor. The contralateral superior thyroid artery did not opacify the tumor (not shown).
In total, contrast enhancement of the entire tumor was not achieved for 6 of 15 hypopharyngeal cancers in group 1 and for 3 of 14 hypopharyngeal cancersin group 2.
Discussion
Head and neck cancers tend to be localized and metastasize to the regional lymph nodes and radiotherapy concomitant with chemotherapy is applied for the purpose of regional tumor control. Intra-arterial chemotherapy has the theoretical advantage of delivering a higher concentration of chemotherapeutic agent to the tumor site and reducing systemic side effects. However, the efficacy of intra-arterial chemotherapy for head and neck cancer is still controversial. Previously, disappointing results of intra-arterial chemotherapy were reported [7, 8] and the efficacy of superselective arterial infusion chemotherapy was also reported [1–4]. A combination of high-dose intra-arterial cisplatin and radiation therapy is effective for improving survival and organ preservation rates in patients with advanced squamous cell carcinoma of the head and neck [9]. During treatment with intra-arterial chemotherapy concomitant with radiotherapy, mucositis to a variable degree is inevitable. However, the rate of serious complications such as neurological deficit in superselective intra-arterial chemotherapy by the Seldinger technique using a microcatheter with a coaxial guiding catheter system is tolerable. We applied this procedure to deal with multiple feeding arteries.
Elucidating the arterial blood supply of a tumor is indispensable for performing efficient and effective intra-arterial infusion chemotherapy. The effectiveness of intra-arterial chemotherapy depends not only on tumor sensitivity but also on the anatomic territory of the infused artery. Lee et al. reported that if the tumor extent is unilaterally contained within the territory of a single external carotid artery, the response rate increases [10]. The normal larynx is primarily perfused by the superior and inferior thyroid arteries, with twigs from the dorsal lingual branch of the lingual artery supplying the epiglottis. It is generally assumed that the bulk of the blood flow to the larynx is delivered via the superior laryngeal artery, the first branch of the superior thyroid artery. In addition to this, the superior thyroid arteries are the dominant arterial supply of the larynx [11] and branches of the lingual artery reach the preepiglottic space and epiglottis through the vallecula [12].
Regarding the arterial supply of the normal hypopharynx, the fronto-lateral divisions of the laryngeal part of the pharynx are supplied by pharyngeal branches of the superior and inferior laryngeal arteries. Also, the posterior wall of the laryngeal part of the pharynx is divided into three zones depending on the main arterial sources: the ascending pharyngeal artery and the superior and inferior thyroid arteries [13].
The superior thyroid artery is typically the first branch of the external carotid artery. It usually arises from the anterior wall of the external carotid artery, coursing anteroinferiorly and slightly medially toward the apex of the thyroid gland. In approximately 20% of cases, the superior thyroid artery arises from the carotid bifurcation. A less common origin of this vessel includes the common carotid (10%) and lingual (2%) arteries [6]. Regarding our patients, the superior thyroid artery arising from the level of the carotid bifurcation was more common (44%) and that from the external and common carotid arteries occurred at frequencies of 46% and 9%, respectively.
The superior thyroid artery supplies the larynx and most of the upper thyroid gland. This vessel has extensive anastomoses with its counterpart from the opposite external carotid artery as well as the inferior thyroid artery, a branch of the thyrocervical trunk [6]. The origin of the superior laryngeal artery is variable. It arises from the superior thyroid artery in about 80% of cases [14]. It might also directly arise from the external carotid artery or the ascending pharyngeal artery. On the other hand, there are several anastomoses between the superior thyroid and the branches of the superior laryngeal artery [5]. In our patients, six superior laryngeal arteries directly arose from the external carotid artery (6%), one from the lingual artery (1%), and the others from the superior thyroid artery (93%). It is essential to identify the superior laryngeal artery arising from the superior thyroid artery for superselective intra-arterial infusion.
The present study showed that for most cases of glottic and supraglottic cancers without or with minimum tumor-spread across the midline, chemotherapeutic agents were delivered via the ipsilateral superior thyroid artery to the entire tumor. Even if a tumor-spread exists across the midline, it can be delivered via the bilateral superior thyroid arteries. As mentioned earlier, it is important to confirm that the superior laryngeal artery arises from the superior thyroid artery. For a few cases in which the superior laryngeal artery arose from the proximal portion of the superior thyroid artery, a microcatheter was unsettled and readily advanced more distal than the origin of the superior laryngeal artery. Catheterization into the superior laryngeal artery was required in such cases. For cases in which the superior laryngeal artery arises from the external carotid artery, direct catheterization into the superior laryngeal artery is also required. Additional intra-arterial infusion via the inferior thyroid artery was not necessary for most cases of laryngeal cancer.
With regard to hypopharyngeal cancer, contrast enhancement of the entire tumor only via the superior thyroid arteries was achieved for a small number of cases. The inferior thyroid artery was confirmed to supply part of the tumor for 41% of the patients with hypopharyngeal cancer. Intra-arterial infusion via the inferior thyroid artery in addition to that via the superior thyroid artery increased the enhanced area within the tumor. Enhancement of the entire tumor in hypopharyngeal cancer occurred at a small ratio compared with that for laryngeal cancer. For four cases of hypopharyngeal cancer involving the piriform sinus, there was a perfusion defect in the posterior portion of the tumor seen by CTSA from the ipsilateral superior thyroid artery. Because the contralateral superior thyroid artery or inferior thyroid artery was not evaluated for those cases, we could not make reference to the tumor location that tended to lack perfusion from the ipsilateral superior thyroid artery. Thus, it might have required more effort to deliver the drug to the entire tumor.
In general, evaluation by CTSA is associated with some technical problems. According to the caliber of the artery, flow rate of injection, and concentration of the contrast media, the area of enhancement might vary, especially as seen by CTSA from the branch that has anastomoses with the contralateral branch or other branches. However, CTSA facilitates the confirmation of feeding arteries of the tumor and the distribution of the drug, even in the deep cervical region. Furthermore, CTSA is easily repeated using a CT with DSA system. CTSA is useful, but it is difficult to perform CTSA without a CT scanner with the DSA system. Such a system has been installed in a limited of hospitals. Overall, information about the arterial blood supply of the head and neck cancer enables efficient and effective intra-arterial chemotherapy.
There are some limitations of this retrospective analysis regarding the accuracy of the perfusion territories. We assumed that the dominant feeding artery was the superior thyroid artery for most cases of laryngeal and hypopharyngeal cancers; therefore, the microcatheter was inserted into it. Also, the feeding artery was determined by CTSA, but we had no strict protocols pertaining to the order or extent of catheterization of the arterial branches. If the entire tumor was enhanced, other branches were not evaluated. However, the purpose of this study was to evaluate the validity of infusion from the superior thyroid artery.
In conclusion, we evaluated the arterial blood supply of head and neck cancers using CTSA. Laryngeal cancer was frequently supplied by the ipsilateral or the bilateral superior thyroid arteries. Superselective intra-arterial chemotherapy for laryngeal cancer from the superior thyroid artery is appropriate. On the other hand, hypopharyngeal cancer is often supplied by the inferior thyroid artery in addition to the superior thyroid artery. To accomplish contrast enhancement of the entire tumor, intra-arterial infusion via other arteries than the superior thyroid artery is necessary.
References
Shimizu T, Sakakura Y, Hattori T, et al. (1990) Superselective intraarterial chemotherapy in combination with irradiation: preliminary report. Am J Otolaryngol 11(2):131–136
Robbins KT, Storniolo AM, Kerber C, et al. (1992) Rapid superselective high-dose cisplatin infusion for advanced head and neck malignancies. Head Neck 14(5):364–371
Kerber CW, Wong WH, Howell SB, et al. (1998) An organ-preserving selective arterial chemotherapy strategy for head and neck cancer. Am J Neuroradiol 19(5):935–941
Korogi Y, Hirai T, Nishimura R, et al. (1995) Superselective intraarterial infusion of cisplatin for squamous cell carcinoma of the mouth: Preliminary clinical experience. Am J Roentgenol 165(5):1269–1272
Lasjaunias P, Berenstein A (1987) Thyrolaryngeal arteries. In: Lasjauuias P, Berenstein A (eds) Surgical Neuroangiography. Volume 1. Functional Anatomy of Craniofacial Arteries. Berlin: Springer-Verlag, pp 207–219
Osborn AG (1999) The external carotid artery. In: Osborn AG (ed) Diagnostic Cerebral Angiography (2nd ed) Philadelphia: Lippincott Williams & Wilkins, pp 31–55
Baker SR, Forastiere AA, Wheeler R, et al. (1987) Intra-arterial chemotherapy for head and neck cancer. An update on the totally implantable infusion pump. Arch Otolaryngol Head Neck Surg 113(11):1183–1190
Muggia FM, Wolf GT (1980) Intraarterial chemotherapy of head and neck cancer: worth another look? Cancer Clin Trials 3(4):375–379
Wilson WR, Siegel RS, Harisiadis LA, et al. (2001) High-dose intra-arterial cisplatin therapy followed by radiation therapy for advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 127(7):809–812
Lee YY, Wallace S, Dimery I, et al. (1986) Intraarterial chemotherapy of head and neck tumors. Am J Neuroradiol 7(2):343–348
Anthony JP, Argenta P, Trabulsy PP, et al. (1996) The arterial anatomy of larynx transplantation: Microsurgical revascularization of the larynx. Clin Anat 9(3):155–159
Pearson BW (1975) Laryngeal microcirculation and pathways of cancer spread. Laryngoscope 85(4):700–713
Sharafislamov FS, Batyrshin RU (1976) [Arterial blood supply to the laryngeal part of the pharynx]. Arkh Anat Gistol Embriol 71(11):45–49
Trotoux J, Germain MA, Bruneau X (1986) [Vascularization of the larynx. Update of classical anatomic data from an anatomical study of 100 subjects]. Ann Otolaryngol Chir Cervicofac 103(6):389–397
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terayama, N., Sanada, J., Matsui, O. et al. Feeding Artery of Laryngeal and Hypopharyngeal Cancers: Role of the Superior Thyroid Artery in Superselective Intraarterial Chemotherapy. Cardiovasc Intervent Radiol 29, 536–543 (2006). https://doi.org/10.1007/s00270-005-0094-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-005-0094-0