Abstract
Purpose
To evaluate the volumetric changes in abdominal aortic aneurysms (AAA) after endovascular AAA repair (EVAR) in 24 months of follow-up.
Methods
We evaluated the volume modifications in 63 consecutive patients after EVAR. All patients underwent strict duplex ultrasound and computed tomography angiography (CTA) follow-up; when complications were suspected, digital subtraction angiography was also performed. CTA datasets at 1, 6, 12, and 24 months were post-processed through semiautomatic segmentation, to isolate the aneurysmal sac and calculate its volume. Maximum transverse diameters (Dmax) were also obtained in the true axial plane, Presence and type of endoleak (EL) were recorded. A statistical analysis was performed to assess the degree of volume change, correlation with diameter modifications, and significance of the volume increase with respect to ELs.
Results
Mean reconstruction time was 7 min. Mean volume reduction rates were 6.5%, 8%, and 9.6% at 6, 12, and 24 months follow-up, respectively. Mean Dmax reduction rates were 4.2%, 6.7%, and 12%; correlation with volumes was poor (r = 0.73–0.81). ELs were found in 19 patients and were more frequent (p = 0.04) in patients with higher preprocedural Dmax, The accuracies of volume changes in predicting ELs ranged between 74.6% and 84.1% and were higher than those of Dmax modifications. The strongest independent predictor of EL was a volume change at 6 months ≤0.3% (p = 0.005), although 6 of 19 (32%) patients with EL showed no significant AAA enlargement, whereas in 6 of 44 (14%) patients without EL the aneurysm enlarged.
Conclusion
The lack of volume decrease in the aneurysm of at least 0.3% at 6 months follow-up indicates the need for closer surveillance, and has a higher predictive accuracy for an endoleak than Dmax.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the last decade, endovascular abdominal aortic aneurysm repair (EVAR) has become a valid alternative to open surgery in selected patients [1–4]. The aim of treatment is to exclude the aneurysm from blood flow, thus preventing rupture [5]. High immediate success rates with low perioperative morbidity and mortality have been demonstrated in several reports [6, 7]. However, as longer follow-up becomes available, several complications have been reported, requiring close surveillance [8, 9], and Computed tomography angiography has been proposed as the best imaging modality to monitor EVAR results [10–13].
Exact definition of the success of the procedure remains a controversial issue [14, 15]. Since aneurysm exclusion is expected to be associated with shrinkage, surveillance of aneurysm dimensions seems to be the most appropriate tool to assess the procedural outcome [14]. Although maximum aneurysm diameter has been used in the majority of initial studies [16, 17], the low reliability of this parameter has been demonstrated, and three-dimensional volume assessment has been proposed as a more valid tool in the early definition of success and in the identification of patients requiring closer surveillance [18–24]. Volume has also been proved to be extremely useful in the decision-making after EVAR, reducing unnecessary and expensive imaging and interventions [25].
The purpose of our study was to retrospectively analyze aneurysm volume modifications after EVAR, to assess the relationship between volume and diameter measurements, and to identify a threshold value of the volume modification rate able to predict the presence of endoleaks (ELs).
Materials and Methods
This retrospective study included 63 patients (male/female = 62/1; mean age 69.8 ± 7.4 years, age range 52–84 years) who underwent EVAR (mean aneurysm diameter 51.8 ± 11.1 mm, range 40–88 mm) using different types of devices. Imaging follow-up was performed by color-coded duplex ultrasound (US; AU5, Esaote Biomedica, Genoa, Italy) at 7 days, 1, 3, 6, 12 months and annually thereafter, and computed tomography angiography (CTA; Highspeed and LightSpeedPlus; GE Medical Systems, Milwaukee, WI, USA) at 1, 6, 12 months and annually thereafter. When complications were suspected, closer surveillance was performed by duplex US and CTA; finally, when required, patients underwent digital subtraction angiography (DSA; Multistar, Siemens, Erlangen, Germany) to confirm the EL and provide adequate treatment.
Mean ± SD follow-up in this series was 43.2 ± 11.6 months (range 24–60 months). However, the present study refers to the changes found in the initial 24 months of follow-up.
CTA Protocol and Post-processing
CTA was performed from the celiac artery to the common femoral arteries both before and after intravenous contrast administration (Visipaque 320; Nycomed, Oslo, Norway) at a dose of 120 ml with a flow rate of 3 ml/sec. Acquisition parameters for spiral CT were: 3 mm collimation, 1 mm reconstruction spacing, and variable pitch. Multidetector CTA was performed with the following parameters: HighSpeed modality, gantry rotation 0.5–0.6, table speed 7.5 mm/rot, 2.5 mm collimation, and 1.2 mm reconstruction algorithm. Both the arterial phase and a delayed phase were acquired. Scan delay ranged between 20 and 40 sec, according to the patient’s circulation time determined by an automated bolus time test (SmartPrep, GE Medical Systems) using 25 ml of iodinated contrast medium. The venous phase was acquired with the same parameters 80 sec after contrast injection.
Images were processed with a dedicated software package on an independent workstation (Advantage Windows 4.1; Sun Microsystems, Mountain View, CA, USA) to generate multiplanar reformations (MPRs), maximum intensity projections (MIPs), and volume renderings (VRs). Volumes were obtained through semiautomatic segmentation, using the summation of area technique. The region of interest was drawn manually at the interface of the outer aortic wall and the extra-aortic tissues, at 5–10 different levels throughout the aorta from the lowest renal artery to the aortic bifurcation. Then, the system automatically determined the boundaries around a class of similar voxel intensity values in the remaining sections and, after proper checking by the operator, the aneurysm sac volume was calculated.
Post-processing and volume assessment of CTA data at 1, 6, 12, and 24 months follow-up were retrospectively performed by a single expert radiologist (I.B.) masked to the follow-up interval and the presence of ELs or other complications. Maximum orthogonal diameter (Dmax) was calculated in the plane perpendicular to the centerline of the aortic lumen. In addition, CTA images were reviewed by consensus by one of several authors (R.C., A.P., P.P., C.V.) to identify the presence of an EL (defined by contrast enhancement within the sac and outside the stent-graft lumen) and of other complications (such as stent-graft migration or disconnection). CTA image analysis was performed without knowledge of the volume measurements.
Duplex US Evaluation
Color-coded duplex US was performed by a single radiologist (V.N.) masked to the CTA findings. The entire abdominal aorta was scanned from the diaphragm to the iliac arteries in the long axis and cross-sectional views with an abdominal phase array (2.5–3.5 MHz), to visualize possible ELs, using color Doppler and power Doppler US imaging and obtaining a spectral Doppler waveform analysis of the detected leaks. In type II ELs, an attempt was made to identify inflow and outflow vessels.
DSA Evaluation
DSA was performed in the angiographic suite, in a standard fashion, by four experienced interventional radiologists (I.B., P.P., R.C., C.V.) aware of the findings of previous examinations. Aortography was obtained (5 Fr Pigtail catheter) with a bolus of 40 ml of iodinated contrast medium (Visipaque 320). Then, selective angiographies were performed according to the aortographic findings, using appropriately shaped catheters. Images were evaluated by consensus to assess presence of an EL (visualized as spreading of contrast material outside the stent-graft) and to identify its origin (from aortic branches or stent-graft disconnections).
Statistical Analysis
Descriptive statistics (proportions, means, SDs) were calculated for patient characteristics and numeric continuous data.
The 1-month CT control represented our baseline for evaluating diameter and volume changes, since it was performed in all patients in our institution according to our protocol. In contrast, preprocedural CT was performed elsewhere in 16 patients in this series. Volume changes were expressed as the percentage of the initial volume at referral represented by the volume calculated at 1-month follow-up (V1), using the following formula: [(V1−Vx)/ V1] × 100. Therefore, a positive value indicated AAA shrinkage, whereas a negative value was indicative of a volume increase. An analogous formula was used to assess Dmax changes. Correlation between volumes and diameters was assessed with the Pearson correlation coefficient and 2 × 2 tables.
By ROC curves, cutoff values were obtained for volume and diameter changes at 6, 12, and 24 months follow-up that best predicted the presence of ELs. Sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, and overall accuracy rates were calculated in predicting ELs.
Volume changes were analyzed with respect to the presence of an EL and the preprocedural diameter by one-way ANOVA, Student’s t, Fisher and χ2 tests.
The analysis also included patient age, type of EL, time of follow-up at which the EL was detected (i.e., primary EL when diagnosed within 1 month after treatment, or secondary EL when diagnosed later).
Stepwise multiple logistic regression analyses were obtained for the evaluation of independent predictors of ELs.
A p value <0.05 was considered statistically significant.
Statistical analysis was performed using JMP software (SAS Institute, Cary, NC, USA).
Results
Patients’ demographic and procedural data are detailed in Table 1.
Mean reconstruction time for each dataset was 7 min (range 5–15 min). Volumes calculated on follow-up ranged from 51 to 529 ml (Fig. 1). Mean ± SD volume reduction rates in the entire population were 6.5 ± 11.1%, 8 ± 14.3%, and 9.6 ± 19.4% at 6, 12, and 24 months follow-up, respectively. The rate of volume change was independent of the preprocedural Dmax. Also patient age was not statistically related to volume changes. Mean ± SD Dmax reduction rates were 4.2 ± 7.6%, 6.7 ± 9.6%, and 12 ± 13.7%, at 6, 12, and 24 months follow-up, respectively. Correlation between Dmax and volume changes was good (r = 0.79, 0.81, 0.73 at 6, 12, and 24 months, respectively).
ELs were detected at CTA and/or duplex US in 19 (31%) patients; 5 cases of type I leak were depicted, due stent-graft disconnection at the proximal (n = 4) or distal (n = 1) neck; the remaining leaks were classified as type II (n = 14), with 7 lumbar and 7 internal mesentric artery (IMA) and lumbar leaks. Nine ELs were detected within the first month of follow-up, whereas the remaining cases were secondary ELs depicted at 3 (n = 1), 6 (n = 4) or 12 (n = 5) months follow-up.
The number of ELs detected in follow-up ranged from 14 at 6 months to 17 at 12 months and 8 at 24 months. ELs were significantly more frequent in patients with higher preprocedural Dmax; the mean Dmax was 56.8 mm in the group of patients with EL and 49.8 mm in patients without EL (p = 0.04) (Table 1).
The type of stent-graft did not affect the presence of perigraft flow, although this is a very inhomogeneous series; even when comparing AneuRx stent-grafts on the one hand with all the other types of endograft on the other, no statistically significant difference was observed in the incidence of ELs (Table 1).
The presence of an EL was significantly associated with the rate of volume change and, to a lesser extent, with the rate of diameter change (Table 2). No statistically significant differences in volume modifications were observed between different types of ELs. In particular, volume changes did not differ significantly between IMA ELs and lumbar ELs. Moreover, the time of EL detection (i.e., primary and secondary ELs) did not affect the rate of volume change.
Using ROC statistics, cutoff values of volume and diameter changes for predicting ELs were assessed (Tables 3, 4), The predictive accuracies of volume changes were higher than those of Dmax modifications, ranging between 74.6% and 84.1% for volumes and between 63.5% and 79.4% for diameters.
When evaluating the cutoff value obtained considering all the ELs independently of their time of detection on follow-up, a change was observed in the threshold values of Dmax at 6 months follow-up (from −1.1% to 4.9%) and of the volume at 24 months follow-up (from 1.1% to −6.7%) (Table 4). Testing the threshold values obtained in Table 3, the sensitivity, specificity, PPV, NPV, and accuracy rates in identifying any EL were 52.6%, 84.1%, 58.8%, 80.4%, and 74.6% for a diameter ≤ −1.1% at 6 months follow-up, and 68.4%, 79.5%, 59.1%, 85.4%, and 76.2% for a volume ≤ 1.1% at 24 months follow-up. Classifying the aneurysms as increased or decreased according to the threshold values specified above, a poor correlation was observed between classifications according to volumes and diameters (κ = −0.14 to 0.66), particularly at 6 months follow-up and in patients with an EL (Fig. 2).
A–D. One month and 6 months CTA follow-up in a patient with primary type II lumbar endoleak. The maximum orthogonal diameter appeared identical 6 months after the procedure (B) compared with the 1 month control (A), whereas the volume measurement demonstrated significant aneurysm enlargement (D), with an increase of 5.23% over the 1 month volume (C) (from 172 to 181 ml). CTA depicted a type II lumbar endoleak.
We tested the 10% threshold value to assess the relationship between a significant volume decrease and the absence of an EL [24–27] and compared the accuracies with those obtained with our own values (Table 5). A low predictive accuracy was obtained due to low sensitivity of the 10% decrease rate, although a high PPV was observed, particularly at 12 and 24 months follow-up.
On the multiple logistic regression analysis, the volume changes at 6 month and 24 months follow-up were both significant independent predictors of an EL (p = 0.005, OR 7.8, 95% CI 0.32–1.78 for the 6 months increase rate ≤ 0.3%; and p = 0.007, OR 12.2, 95% CI 0.4–2.3 for the 24 months increase rate ≤−6.7%).
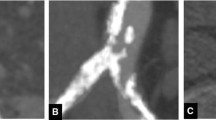
Using the 6 months increase rate ≤ 0.3%, we observed 6 patients without an EL at any time in whom the aneurysm volume either remained stable (n = 1) or increased (n = 5). In contrast, in 6 cases with evidence of an EL, no significant volume enlargement was observed at 6 months follow-up (Table 6). These cases included 3 type I ELs (2 primary and 1 secondary ELs) and 3 type II ELs (1 primary and 2 secondary ELs). Nevertheless, 4 of 6 patients showed enlargement later on follow-up. Among patients with type I EL, one case (#3) was treated immediately by an extensive cuff, with no volume increase; another case (#1) was initially misdiagnosed as a type II EL and treated as soon as the volume enlarged. The last patient with primary type I EL (#5) did not show any aneurysm enlargement; an excessive oversizing of the proximal neck had caused the stent-graft to fold at this level, with incomplete sealing to the aortic wall and presence of a posterior EL; nevertheless, the EL was no longer depicted at 6 months follow-up and later the aneurysm progressively shrank (Fig. 3).
A–E. One month and 24 months CTA follow-up in a patient with primary type I endoleak. The 1 month CT control showed an incorrect deployment of the stent-graft that was folded at the level of the proximal anchorage (A), with a proximal endoleak caused by the incomplete sealing of the stent-graft to the aortic wall (B, arrow). The endoleak was no longer visible 6 months after EVAR (C); the 24 months volume measurement (E) demonstrated a significant reduction of the aneurysmal sac by 46% relative to the 1 month control volume (D) (from 200 to 108 ml) confirming the exclusion of the aneurysm from blood flow.
Discussion
EVAR has become an appealing alternative to open surgery in patients with AAA [1–4]. Its high immediate technical success and low perioperative morbidity and mortality have been proved in several studies [6, 7]. Nonetheless, concern has been expressed regarding the incidence of mid- and long-term complications [9], such as continued pressurization of the sac through ELs, which might occur in up to 45% of patients, and stent-graft complications, due to disconnections and material consumption, with the frequent need for secondary interventions [28, 29]. Therefore strict surveillance is needed, with an attendant increase in postoperative costs [7, 30].
CTA represents the best imaging modality in the follow-up of patients after EVAR, allowing assessment of both aneurysm and endograft morphology [10–13, 24]. Since aneurysmal sac reduction represents the main goal of treatment, several early reports have focused on maximum aneurysm diameter changes [16, 17]. However, recent studies have questioned the reliability of this parameter in the assessment of EVAR results, and more detailed three-dimensional reconstruction methods have been proposed [18, 22–31]. In fact, several studies have proved volume assessment to be more accurate than diameter in the early detection of aneurysm growth [23], allowing efficient patient management and avoiding unnecessary, risky, and expensive examinations and interventions, particularly in the first 12-months of follow-up [25].
We analyzed AAA volume changes over 24 months of follow-up in a series of 63 consecutive patients and correlated these modifications with diameter changes and with the presence of ELs. In our series, 19 ELs (30%) were detected over the entire follow-up period, with a higher incidence in patients with a higher preprocedural aneurysm diameter [32]. In the entire series, volumes and diameters decreased during follow-up, with a statistically significant difference of both parameters in patients with or without ELs [16, 17, 33]. Yet, volumes seemed to be more efficient in the early detection of ELs, with a higher area under the curve at ROC analysis [20]. In fact, diameter changes seem to occur later in follow-up [20, 34], and the best correlation between diameter and presence of ELs was obtained 24 months after the procedure.
Moreover, our data showed a relatively low correlation between the rates of change of volume and diameter (r = 0.73–0.81). Up to 37% of patients with increasing aneurysm volumes showed decreases in aneurysm diameter, particularly at 6 months follow-up and in patients with EL. This phenomenon could cause underestimation of the importance of persisting flow within the sac and, therefore, of the risk of rupture. Diameter is of course the expression of aneurysm changes in only a single cross-section, whereas volume changes involve the aneurysm as a whole [18, 19, 33]. Changes in aneurysm morphology can alter the level corresponding to the maximum diameter at each CTA examination. In addition, the true axial plane perpendicular to the centerline length might change over time, and operator variability seems to be greater in diameter measurement than in the volume assessment [27, 35].
However, volume assessment is time-consuming and requires advanced processing, dedicated equipment, and skilled operators [21, 25, 26]. Several specific softwares have recently been introduced to reduce processing time and variability [36]. We used a semiautomatic segmentation technique [21], and all volumes were measured by a single masked operator. Reconstruction time was therefore usually less than 10 min, which in our experience is an acceptable time for CTA data analysis. Measurement variability was limited by the use of a single operator [27] and of semiautomatic segmentation instead of a fully manual technique [21, 33, 36, 37]. Nonetheless, intraobserver variability was not analyzed in our study; we obtained a cutoff referral value by ROC curves and compared our predictive accuracies with results obtained using the cutoff values proposed in other reports [23, 25, 27].
Our threshold values were much lower than the 10% decrease proposed by other authors, according to the reproducibility coefficient [27], although recent studies have reported lower intraobserver variabilities (1.4–3%) using automatic software [36, 38]. A possible explanation for this evident difference might be the relatively higher number of patients in our study with aneurysms only 4–5 cm in diameter. Other studies have focused on patients with AAA at least 6 cm in diameter, and therefore a higher preprocedural volume [25], that might undergo a more evident decrease at 6 months follow-up. However, no data are presently available, nor in our series could a relationship be demonstrated between the volume reduction rate and preprocedural diameter.
According to our data, the use of a 10% volume change is associated with a higher specificity but much lower sensitivity than the 0.3% modification rate at 6 months follow-up. Therefore, underestimation of volume increase might occur. Our predictive values in the detection of ELs compare favorably with the published data, with over 80% specificity, sensitivity, and overall accuracy rates [23]. The strongest predictor of an EL was lack of a volume decrease of at least 0.3% at 6 months follow-up, which had the ability to predict any EL, independent of its detection time. This value points out the importance not only of enlarging aneurysms, but also of almost stable sacs that might be associated with persistent flow and pressurization. Therefore, stable volumes might represent an imminent EL and might require closer surveillance [23, 25, 39].
The existence of patients with an EL and no aneurysm enlargement should be evaluated carefully. We observed 6 ELs in patients with a decrease in aneurysm volume 6 months after EVAR; however, in the majority of cases the volume increased later on follow-up [31], except for 2 cases of type I proximal EL.
No statistically significant differences in volume modification rates were observed on comparing different types of ELs. However, we agree with the general concern expressed regarding the rapid growth caused by type I ELs [41], which requires immediate treatment, and our results could be biased by the immediate successful treatment of type I ELs in the vast majority of our patients.
Six cases of aneurysm increase with no evidence of complications at duplex US and CTA were observed in our series. The term “endotension” has been proposed in these patients, although its nature is still a debated topic [14, 40]. In fact, endotension might be the expression of a nonvisualized EL [41], and alternative imaging modalities have been proposed as more sensitive tools in detecting perigraft flow, such as magnetic resonance angiography [42] and contrast-enhanced US [43, 44].
The present study is limited by its retrospective design. Nonetheless, volume analysis was performed without knowledge of patient follow-up and outcome, and the presence of complications at CTA and duplex US was assessed by different radiologists, masked to the volume measurements. Moreover, only a small number of patients was included in the present study with a relatively short follow-up. However, all patients had identical continuous follow-up with both CTA and duplex US [11].
We used the presence or absence of an EL as the gold standard to evaluate the significance of aneurysm changes. Probably, other parameters, such as the intrasac pressure measurement, would be more appropriate [45]; however, for ethical reasons this parameter was not available in our series.
Nonetheless, our findings are substatially in agreement with prevous studies [23, 25], confirming the importance of volume measurement, particularly in the short-term follow-up, to avoid unnecessary imaging and interventions. It might be a time-consuming process, yet it should be performed in all patients at 1 and 6 months follow-up, whenever an EL is detected, to evaluate its significance on AAA pressurization, and when the diameter appears persistently stable on follow-up. The lack of a volume decrease of at least 0.3% at 6 months follow-up indicates the need for closer surveillance, and has a high predictive accuracy for the presence of complications.
References
JC Parodi JC Palmaz HD Barone (1991) ArticleTitleTransfemoral intraluminal graft implantation for abdominal aortic aneurysms Ann Vasc Surg 5 491–499 Occurrence Handle1:STN:280:DyaK387is1Cltw%3D%3D Occurrence Handle10.1007/BF02015271 Occurrence Handle1837729
LH Walschot RJ Laheij AL Verbeek (2002) ArticleTitleOutcome after endovascular abdominal aortic aneurysm repair: A meta-analysis J Endovasc Ther 9 82–89 Occurrence Handle10.1177/152660280200900114 Occurrence Handle11958330
CK Zarins RA White FL Moll et al. (2001) ArticleTitleThe AneuRx stent graft: Four-year results and worldwide experience 2000 J Vasc Surg 33 IssueID2 Suppl S135–S145 Occurrence Handle1:STN:280:DC%2BD3M7lslynuw%3D%3D Occurrence Handle10.1067/mva.2001.111676 Occurrence Handle11174825
ML Marin LH Hollier SH Ellozy et al. (2003) ArticleTitleEndovascular stent graft repair of abdominal and thoracic aortic aneurysms: A ten-year experience with 817 patients Ann Surg 238 586–593 Occurrence Handle14530730 Occurrence Handle1360117
TAM Chuter K Ivancev M Malina et al. (1996) ArticleTitleAneurysm pressure following endovascular exclusion Eur J Vasc Endovasc Surg 13 85–87 Occurrence Handle10.1016/S1078-5884(97)80056-1
S Elkouri P Gloviczki MA McKusick et al. (2004) ArticleTitlePerioperative complications and early outcome after endovascular and open surgical repair of abdominal aortic aneurysms J Vasc Surg 39 497–505 Occurrence Handle10.1016/j.jvs.2003.10.018 Occurrence Handle14981437
WA Lee JW Carter G Upchurch et al. (2004) ArticleTitlePerioperative outcomes after open and endovascular repair of intact abdominal aortic aneurysms in the United States during 2001 J Vasc Surg 39 491–496 Occurrence Handle10.1016/j.jvs.2003.12.001 Occurrence Handle14981436
MA Corriere ID Feurer SY Becker et al. (2004) ArticleTitleEndoleak following endovascular abdominal aortic aneurysm repair: Implications for duration of screening Ann Surg 239 800–807 Occurrence Handle10.1097/01.sla.0000128300.60156.ab Occurrence Handle15166959 Occurrence Handle1356288
GA Fransen SR Vallabhaneni SuffixSr CJ Marrewijk Particlevan et al. (2003) ArticleTitleRupture of infta-renal aortic aneurysm after endovascular repair: A series from EUROSTAR registry Eur J Vasc Endovasc Surg 26 487–493 Occurrence Handle1:STN:280:DC%2BD3svntVWntg%3D%3D Occurrence Handle10.1016/S1078-5884(03)00350-2 Occurrence Handle14532875
J Golzarian L Dussaussois HT Abada et al. (1998) ArticleTitleHelical CT of aorta after endoluminal stent-graft therapy: Value of biphasic acquisition AJR Am J Roentgenol 171 329–331 Occurrence Handle1:STN:280:DyaK1czlslyiug%3D%3D Occurrence Handle10.2214/ajr.171.2.9694445 Occurrence Handle9694445
S Thurnher M Cejna (2002) ArticleTitleImaging of aortic stent-grafts and endoleaks Radiol Clin North Am 40 799–833 Occurrence Handle10.1016/S0033-8389(02)00024-6 Occurrence Handle12171186
AM Rozenblit M Patlas AT Rosenbaum et al. (2003) ArticleTitleDetection of endoleaks after endovascular repair of abdominal aortic aneurysm: Value of unenhanced and delayed Helical CT acquisitions Radiology 227 426–433 Occurrence Handle10.1148/radiol.2272020555 Occurrence Handle12676973
KG Raman N Missig-Carroll T Richardson et al. (2003) ArticleTitleColor-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair J Vasc Surg 2003 38 645–651
GL Gilling-Smith J Martin S Sudhindran et al. (2000) ArticleTitleFreedom from endoleak after endovascular aneurysm repair does not equal treatment success Eur J Vasc Endovasc Surg 19 421–425 Occurrence Handle1:STN:280:DC%2BD3c3msVWisg%3D%3D Occurrence Handle10.1053/ejvs.1999.1029 Occurrence Handle10801377
M Mehta FJ Veith T Ohki et al. (2003) ArticleTitleSignificance of endotension, endoleak, and aneurysm pulsatility after endovascular repair J Vasc Surg 37 842–846 Occurrence Handle10.1067/mva.2003.183 Occurrence Handle12663987
MC Farner JP Carpenter RA Baum et al. (2003) ArticleTitleEarly changes in abdominal aortic aneurysm diameter after endovascular repair J Vasc Interv Radiol 14 205–210 Occurrence Handle10.1097/01.RVI.0000058322.82956.ad Occurrence Handle12582188
YG Wolf BB Hill GD Rubin et al. (2000) ArticleTitleRate of change in abdominal aortic aneurysm diameter after endovascular repair J Vasc Surg 32 108–115 Occurrence Handle1:STN:280:DC%2BD3czjs1Oktg%3D%3D Occurrence Handle10.1067/mva.2000.107754 Occurrence Handle10876211
R Balm R Kaatee JD Blankensteijn et al. (1996) ArticleTitleCT-angiography of abdominal aortic aneurysms after transfemoral endovascular management Eur J Vasc Endovasc Surg 12 182–188 Occurrence Handle1:STN:280:DyaK28zksl2gsw%3D%3D Occurrence Handle10.1016/S1078-5884(96)80104-3 Occurrence Handle8760980
MP Armon SW Yusuf SC Whitaker et al. (1998) ArticleTitleThrombus distribution and changes in aneurysm size following endovascular aortic aneurysm repair Eur J Vasc Endovasc Surg 16 472–476 Occurrence Handle1:STN:280:DyaK1M7htFymtA%3D%3D Occurrence Handle10.1016/S1078-5884(98)80236-0 Occurrence Handle9894485
JJ Wever JD Blankensteijn M WP Th Mali et al. (2000) ArticleTitleMaximal aneurysm diameter follow-up is inadequate after endovascular abdominal aortic aneurysm repair Eur J Vasc Endovasc Surg 20 177–182 Occurrence Handle1:STN:280:DC%2BD3cvjtlOhtQ%3D%3D Occurrence Handle10.1053/ejvs.1999.1051 Occurrence Handle10942691
BV Czermak G Fraedrich MF Schocke et al. (2001) ArticleTitleSerial CT volume measurements after endovascular aortic aneurysm repair J Endovasc Ther 8 380–389 Occurrence Handle1:STN:280:DC%2BD3Mrgt1Kruw%3D%3D Occurrence Handle10.1177/152660280100800407 Occurrence Handle11552730
B Kritpracha HG Beebe AJ Comerota (2004) ArticleTitleAortic diameter is an insensitive measurement of early aneurysm expansion after endografting J Endovasc Ther 11 184–190 Occurrence Handle10.1583/03-976.1 Occurrence Handle15056034
JT Lee IN Aziz JT Lee et al. (2003) ArticleTitleVolume regression of abdominal aortic aneurysms and its relation to successful endoluminal exclusion J Vasc Surg 38 1254–1263 Occurrence Handle10.1016/S0741-5214(03)00924-8 Occurrence Handle14681624
RA White CE Donayre I Walot et al. (2001) ArticleTitleComputed tomography assessment of abdominal aortic aneurysm morphology after endograft exclusion J Vasc Surg 33 S1–S10 Occurrence Handle1:STN:280:DC%2BD3M7lslynsw%3D%3D Occurrence Handle10.1067/mva.2001.111680 Occurrence Handle11174806
M Prinssen EL Verhoeven HJ Verhagen et al. (2003) ArticleTitleDecision-making in follow-up after endovascular aneurysm repair based on diameter and volume measurements: A blinded comparison Eur J Vasc Endovasc Surg 26 184–187 Occurrence Handle1:STN:280:DC%2BD3szotFGksQ%3D%3D Occurrence Handle10.1053/ejvs.2002.1892 Occurrence Handle12917836
YG Wolf M Tillich WA Lee et al. (2002) ArticleTitleChanges in aneurysm volume after endovascular repair of abdominal aortic aneurysm J Vasc Surg 36 305–309 Occurrence Handle10.1067/mva.2002.126085 Occurrence Handle12170211
JJ Wever JD Blankensteijn JC Rijn Particlevan et al. (2000) ArticleTitleInter- and intraobserver variability of CT measurements obtained after endovascular repair of abdominal aortic aneurysms AJR Am J Roentgenol 175 1279–1282 Occurrence Handle1:STN:280:DC%2BD3M%2FisVOqtA%3D%3D Occurrence Handle10.2214/ajr.175.5.1751279 Occurrence Handle11044022
FJ Veith RA Baum T Ohki et al. (2002) ArticleTitleNature and significance of endoleaks and endotension: Summary of opinions expressed at an international conference J Vasc Surg 35 1029–1035 Occurrence Handle10.1067/mva.2002.123095 Occurrence Handle12021724
ES Sampram MT Karafa EJ Mascha et al. (2003) ArticleTitleNature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm J Vasc Surg 37 930–937 Occurrence Handle10.1067/mva.2003.281 Occurrence Handle12756335
Prinssen M, Wixon CL, Buskens E, et al (2004) Surveillance after endovascular aneurysm repair: Diagnostics, complications, and associated costs. Ann Vasc Surg 18:421–427
JG Pollock SJ Travis SC Whitaker et al. (2002) ArticleTitleEndovascular AAA repair: Classification of aneurysm sac volumetric change using spiral computed tomographic angiography J Endovasc Ther 9 185–193 Occurrence Handle10.1177/152660280200900208 Occurrence Handle12010098
CB Rockman PJ Lamparello MA Adelman et al. (2002) ArticleTitleAneurysm morphology as a predictor of endoleak following endovascular aortic aneurysm repair: do smaller aneurysm have better outcomes? Ann Vasc Surg 16 644–651 Occurrence Handle10.1007/s10016-001-0200-y Occurrence Handle12183772
R Singh-Ranger T McArthur MD Corte et al. (2000) ArticleTitleThe abdominal aortic aneurysm sac after endoluminal exclusion: A medium-term morphologic follow-up based on volumetric technology J Vasc Surg 31 490–500 Occurrence Handle1:STN:280:DC%2BD3c7nsFKlsA%3D%3D Occurrence Handle10.1067/mva.2000.102592 Occurrence Handle10709062
M Prinssen JD Blankensteijn (2002) ArticleTitleThe sac shrinking process after EAR does not start immediately in most patients Eur J Vasc Endovasc Surg 23 426–430 Occurrence Handle1:STN:280:DC%2BD383ovVeltw%3D%3D Occurrence Handle10.1053/ejvs.2002.1605 Occurrence Handle12027470
NS Cayne FJ Veith EC Lipsitz et al. (2004) ArticleTitleVariability of maximal aortic aneurysm diameter measurements on CT scan: Significance and methods to minimize J Vasc Surg 39 811–815 Occurrence Handle10.1016/j.jvs.2003.11.042 Occurrence Handle15071447
KK Yeung MJ Laan Particlevan der JJ Wever et al. (2003) ArticleTitleNew post-imaging software provides fast and accurate volume data from CTA surveillance after endovascular aneurysm repair J Endovasc Ther 10 887–893 Occurrence Handle10.1177/152660280301000507 Occurrence Handle14656186
S Nawaratne R Fabiny JE Brien et al. (1997) ArticleTitleAccuracy of volume measurement using helical CT J Comput Assist Tomogr 21 481–486 Occurrence Handle1:STN:280:DyaK2s3otFemtw%3D%3D Occurrence Handle10.1097/00004728-199705000-00027 Occurrence Handle9135663
NJ Aarts GW Schurink U Schultze Kool et al. (1999) ArticleTitleAbdominal aortic aneurysm measurements for endovascular repair: Intra- and interobserver variability of CT measurements Eur J Vasc Endovasc Surg 18 475–480 Occurrence Handle1:STN:280:DC%2BD3c7gsFKgsA%3D%3D Occurrence Handle10.1053/ejvs.1999.0883 Occurrence Handle10637142
T Resch K Ivancev J Brunkwall et al. (2000) ArticleTitleMidterm changes in aortic aneurysm morphology after endovascular repair J Endovasc Ther 7 279–285 Occurrence Handle1:STN:280:DC%2BD3M%2FltVCisw%3D%3D Occurrence Handle10.1177/152660280000700404 Occurrence Handle10958291
GH White J May P Petrasek et al. (1999) ArticleTitleEndotension: An explanation for continued AAA growth after successful endoluminal repair J Endovasc Surg 6 308–315 Occurrence Handle1:STN:280:DC%2BD3czkvFaiuw%3D%3D Occurrence Handle10.1583/1074-6218(1999)006<0308:EAEFCA>2.0.CO;2 Occurrence Handle10893130
GH White (2001) ArticleTitleWhat are the causes of endotension? J Endovasc Ther 8 454–456 Occurrence Handle1:STN:280:DC%2BD3MnntF2htA%3D%3D Occurrence Handle10.1177/152660280100800505 Occurrence Handle11718402
L Engellau U Albrechtsson S Hojgard et al. (2003) ArticleTitleCosts in follow-up of endovascularly repaired abdominal aortic aneurysms: Magnetic resonance imaging with MR angiography versus EUROSTAR protocols Int Angiol 22 36–42 Occurrence Handle1:STN:280:DC%2BD3s3kslKisA%3D%3D Occurrence Handle12771854
PJ Bendick PG Bove GW Long GB Zelenock OW Brown CJ Shanley (2003) ArticleTitleEfficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts J Vasc Surg 37 381–385 Occurrence Handle10.1067/mva.2003.17 Occurrence Handle12563210
Napoli V, Bargellini I, Sardella SS, et al (2004) Contrast-enhanced ultrasound in missed endoleaks after endoluminal repair of abdominal aortic aneurysms. Radiology 233:217–225
B Sonesson N Dias M Malina P Olofsson et al. (2003) ArticleTitleIntra-aneurysm pressure measurements in successfully excluded abdominal aortic aneurysm after endovascular repair J Vasc Surg 37 733–738 Occurrence Handle10.1067/mva.2003.138 Occurrence Handle12663970
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bargellini, I., Cioni, R., Petruzzi, P. et al. Endovascular Repair of Abdominal Aortic Aneurysms: Analysis of Aneurysm Volumetric Changes at Mid-Term Follow-Up. Cardiovasc Intervent Radiol 28, 426–433 (2005). https://doi.org/10.1007/s00270-004-0171-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-004-0171-9