Abstract
We report on a case of a wide-necked aneurysm of the pancreatico-duodenal artery with occlusion of the celiac trunk in an asymptomatic patient. The aneurysm was considered to be at high risk of rupture. Successful embolization after interdisciplinary consultation was followed with color-coded duplex ultrasound (CCDS) demonstrating significant flow reduction. Three weeks later CCDS and angiography demonstrated exclusion of the aneurysm and a patent arterial supply of the liver and spleen fed by superior mesenteric artery (SMA) collaterals. The patient has done well so far, without major adverse clinical events or evidence for tissue necrosis of the liver, pancreas or spleen. Discussion of the case and review of the literature indicate that transcatheter embolization is the therapy of choice even in complicated cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case Report
We present the case of a 70-year-old woman who was referred by her general practitioner. The patient, who was otherwise in good health, had undergone a check-up abdominal ultrasound examination which revealed a circular, unechogenic lesion within the pancreatic head. CCDS identified the lesion as an aneurysm with arterial flow signals in the aneurysm sac. The patient was referred to our vascular imaging unit for further diagnosis and eventual therapy.
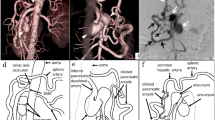
Digital subtraction angiography (DSA) and CT angiography (CTA) revealed complete occlusion of the celiac trunk at its origin. The prominent superior mesenteric artery (SMA) fed the pancreatico-duodenal artery (PDA) in retrograde direction. The latter vessel showed a wide-necked aneurysm of about 2.4 × 1.7 × 2.4 cm (sagittal × cranio-caudal × posteroanterior) protruding into the uncinate process of the pancreatic head. Based on the imaging findings and the clinical history with an episode of pancreatitis, this was considered to be most likely a flow-related aneurysm due to celiac trunk occlusion of unknown etiology. The aneurysm might have caused an episode of pancreatitis. A false aneurysm being caused by pancreatitis was considered as a possible differential diagnosis. The common hepatic artery and its branches were comparatively small, while the splenic artery was wide (Figs. 1, 2). After interdisciplinary discussion with vascular and abdominal surgeons, angiologists and interventional radiologists, transcatheter embolization was favored as treatment option despite the disadvantageous anatomy of the aneurysm that lacked a defined neck. Surgery was deemed risky, since the broad base of the aneurysm would make exclusion difficult. Implantation of a stent-graft was discussed as an option; however, the extremely tortuous vessel anatomy would have made placement most likely impossible. Thus the therapeutic strategy was to reduce the flow in the aneurysm itself by packing it with coils, causing thrombotic occlusion of the aneurysm sac.
Selective digital subtraction angiography of the superior mesenteric artery showing the broad-based aneurysm of the retrogradely perfused pancreatico-duodenal artery (PDA). The celiac trunk is completely occluded. Note the small superior mesenteric artery (SMA) branch (arrowhead) also feeding the PDA.
Catheterization of the PDA was expectedly difficult. The tortuous vessel course with several hairpin bends precluded crossing the aneurysm with a guidewire or catheter, thus preventing the occlusion of the inflow and outflow vessels or the placement of an embolism protection device or a stent-graft. After having successfully placed a 5 Fr cobra catheter (Radifocus Glidecath C2, Terumo, Japan) over a 0.035-inch guidewire (Radifocus Guidewire M, Terumo, Japan) in the aneurysm sac it was carefully filled with 25 embolization coils of 5–12 mm coil diameter (MReye Embolization Coils, Cook Europe, Denmark) (Fig. 3). Final angiography demonstrated markedly reduced flow in the aneurysm while the hepatic and splenic arteries were still patent. The patient remained in our clinic for 3 more days to enable us to control the process of aneurysm thrombosis as well as to watch for signs of hepatic or splenic ischemia or ischemic cholangitis, which did not occur. At discharge, CCDS showed minimal residual perfusion of the aneurysm sac. Three weeks later, CCDS demonstrated complete occlusion of the aneurysm. Four weeks later a follow-up DSA confirmed the CCDS-findings. The aneurysm itself and the short connecting branch to the SMA were completely occluded. The PDA distal to the aneurysm remained patent via a small collateral vessel from the SMA, thus maintaining the arterial supply of the liver and the spleen. There was no retrograde perfusion into the aneurysm sac. The patient is still under review in the outpatient clinic and further follow-up at 12-month intervals is planned.
Discussion
Aneurysms of the splanchnic vessels are an uncommon finding, and less than 1% of these aneurysms are located in the PDA [1, 2]. Atherosclerosis is considered to be the most common underlying cause; other etiologic factors such as pancreatitis, fibrodysplasia, trauma or congenital anomalies may play a significant role. [3, 4].
In up to 80% of all cases of PDA aneurysms an occlusion of the celiac trunk has been reported [2, 5–8]. As a consequence, blood flow in the SMA is increased, which results in greater wall stress that is presumably responsible for aneurysm formation.
Symptoms of splanchnic aneurysms are nonspecific and comprise abdominal discomfort, epigastric pain, nausea and occasional intestinal angina. Some patients remain completely asymptomatic. Most aneurysms are detected incidentally through a diagnostic investigation of nonspecific abdominal pain. Frequently the first manifestation is hemorrhagic shock upon rupture. Despite their unknown natural history, treatment of large aneurysms is thought to be mandatory due to the known high mortality following rupture. There are, however, no hard data available on the rupture frequency in relation to aneurysm size and location.
Duplex ultrasound, CTA and/or MR angiography (MRA) contribute to the diagnosis, but DSA remains essential to assess the origin of the aneurysm and to delineate its neck.
Several publications [6–13] reflect growing experience in percutaneous embolotherapy of visceral aneurysms during the past decade. However, these reports provide no information about the morphology of the aneurysm neck. Percutaneous occlusion of wide-necked aneurysms can be achieved by occlusion of both the inflow and outflow segment of the vessel. If such an occlusion is not possible, “packing” the aneurysm itself with coils causing thrombotic occlusion may be considered as an option. Embolization material for high-flow aneurysms, especially those with a wide neck, should be limited to coils with a diameter exceeding that of the outflow artery to prevent embolization and focal necrosis in the liver or spleen. Embolism protection devices placed in the outflow vessel can prevent this; however, depending on the course and anatomy of the vessel, placement of these devices is not always possible. Stent-grafts have also been reported to achieve successful exclusion, if vascular anatomy allows for placement of such a graft [9, 13].
In the present case, we decided to attempt transcatheter embolization despite the disadvantageous anatomy, since surgical ligature was rated to be very difficult. Coll et al. reviewed all PDA cases treated since 1980 [8]: Forty-two cases were reported (25 surgical patients, 13 embolization patients, 4 untreated patients), with an overall in-hospital mortality rate of 17%. Stratified by therapy the operative mortality was 20% (5/25 cases) compared with 0 for the endovascular group (0/14 cases). Embolization was successfully performed in 86% (12/14 cases). The risk of reperfusion of the aneurysm and recurrent hemorrhage are similar following both surgical and interventional treatment.
Regardless of the etiology, embolotherapy seems to provide excellent long-term results [6–8, 10]. There is, however, little experience in the embolotherapy of wide-necked aneurysms. This situation carries an increased risk of embolic hepatic or splenic artery branch occlusion through coil dislocation. With additional occlusion of the celiac trunk, there is an increased risk of ischemic damage to the liver and spleen following interruption of the retrograde perfusion of the common hepatic artery and the splenic artery. However, transarterial chemo-embolization (TACE) of malignant liver tumors has demonstrated that the liver can tolerate occlusion even of the common hepatic artery as long as the portal vein is fully patent. In such cases, patency of the portal vein must be proven before intervention through either CCDS, CT or indirect splenoportography. The patient also needs to be controlled for signs of ischemic cholangitis rarely occurring after sudden occlusion of the hepatic artery. In rare cases, an aortohepatic bypass operation may be considered to protect the liver.
Cases of splenic artery occlusion for treatment of hypersplenism and traumatic lesions of the spleen show that splenic function may be partially reduced but complete necrosis is rare [14, 15]. Even if acute splenic infarction occurs, splenectomy is considered to have a lower operative risk than the surgical treatment of the aneurysm.
Although transcatheter embolization is now the treatment of choice for visceral artery aneurysms, the potential complications of embolotherapy need to be carefully weighed against the increased risk of surgery and of rupture. In the individual patient, the indication for treatment of aneurysms in this anatomically complex region requires an interdisciplinary approach. Choice of the best treatment and planning of the intervention requires utilization of the entire range of diagnostic modalities to ensure the best possible depiction of the situation including morphologic (CTA/MRA, DSA) and functional (CCDS) assessment. The intervention should be carried out in specialized centers with the equipment to handle possible complications and by an interventionist experienced in vascular interventions and embolotherapy. If these conditions are met, even complicated cases such as the one presented can be successfully treated by transcatheter embolization.
References
JD Mora (1976) ArticleTitleCoeliac-axis artery stenosis with aneurysmal calcification of the collateral supply Australas Radiol 20 252–254 Occurrence Handle1:STN:280:DyaE2s7ls12ktg%3D%3D Occurrence Handle10.1111/j.1440-1673.1976.tb02032.x
AF White S Baum S Buranasiri (1976) ArticleTitleAneurysms secondary to pancreatitis AJR Am J Roentgenol 127 393–396 Occurrence Handle1:STN:280:DyaE283nsFGgug%3D%3D Occurrence Handle10.2214/ajr.127.3.393
S Kadir CA Athanasoulis Hy Yune et al. (1978) ArticleTitleAneurysms of the pancreaticoduodenal arteries in association with celiac axis occlusion Cardiovasc Radiol 1 173–177 Occurrence Handle1:STN:280:DyaE1M7isF2huw%3D%3D Occurrence Handle10.1007/BF02552029
G Proud J Chamberlain (1978) ArticleTitleAneurysm formation on the small pancreatic arteries in association with coeliac axis compression Ann R Coll Surg Engl 60 294–297 Occurrence Handle1:STN:280:DyaE1c3gvFaqsA%3D%3D Occurrence Handle666233 Occurrence Handle2492113
LM Graham JC Stanley Wm Whitehouse SuffixJr et al. (1985) ArticleTitleCeliac artery aneurysms: historic (1745–1949) versus contemporary (1950–1984) differences in etiology and clinical importance J Vasc Surg 2 757–764 Occurrence Handle1:STN:280:DyaL2M3ptlCjsw%3D%3D Occurrence Handle10.1016/0741-5214(85)90053-9
SR Mandel PF Jaques S Sanofsky et al. (1987) ArticleTitleNonoperative management of peripancreatic arterial aneurysms. A 10-year experience Ann Surg 205 126–128 Occurrence Handle1:STN:280:DyaL2s7islSrsg%3D%3D Occurrence Handle10.1097/00000658-198702000-00004
P Quandalle JP Chambon P Marache et al. (1990) ArticleTitlePancreaticoduodenal artery aneurysms associated with celiac axis stenosis: Report of two cases and review of the literature Ann Vasc Surg 4 540–545 Occurrence Handle1:STN:280:DyaK3M%2FosVahtg%3D%3D Occurrence Handle10.1016/S0890-5096(06)60835-2
DP Coll R Ierardi MD Kerstein et al. (1998) ArticleTitleAneurysms of the pancreaticoduodenal arteries: A change in management Ann Vasc Surg 12 286–291 Occurrence Handle1:STN:280:DyaK1c3ksl2gsA%3D%3D Occurrence Handle10.1007/s100169900155
U Nyman P Svendsen L Jivegard et al. (2000) ArticleTitleMultiple pancreaticoduodenal aneurysms: treatment with superior mesenteric artery stent-graft placement and distal embolization J Vasc Interv Radiol 11 1201–1205 Occurrence Handle1:STN:280:DC%2BD3cvptVajtA%3D%3D Occurrence Handle10.1016/S1051-0443(07)61364-5
A Gabelmann J Gorich EM Merkle (2002) ArticleTitleEndovascular treatment of visceral artery aneurysms J Endovasc Ther 9 38–47 Occurrence Handle11958324
S Sultan M Molloy D Evoy et al. (2002) ArticleTitleEndovascular management of a pancreaticoduodenal aneurysm: A clinical dilemma J Endovasc Ther 9 225–228 Occurrence Handle10.1177/152660280200900216
BG Peterson SA Resnick MK Eskandari (2003) ArticleTitleCoil embolization of an inferior pancreaticoduodenal artery aneurysm associated with celiac artery occlusion Cardiovasc Surg 11 515–519 Occurrence Handle10.1016/S0967-2109(03)00131-5
H Ogino T Banno Y Sato et al. (2004) ArticleTitleSuperior mesenteric artery stent-graft placement in a patient with pseudoaneurysm developing from a pancreatic pseudocyst Cardiovasc Intervent Radiol 27 68–70 Occurrence Handle1:STN:280:DC%2BD2c3htFensw%3D%3D Occurrence Handle10.1007/s00270-003-2727-5
JM Haan W Biffl MM Knudson et al. (2004) ArticleTitleSplenic embolization revisited: A multicenter review J Trauma 56 542–547 Occurrence Handle10.1097/01.TA.0000114069.73054.45
F Kimura H Itoh S Ambiru et al. (2002) ArticleTitleLong-term results of initial and repeated partial splenic embolization for the treatment of chronic idiopathic thrombocytopenic purpura AJR Am J Roentgenol 179 1323–1326 Occurrence Handle10.2214/ajr.179.5.1791323
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber, C., Pfeifer, K., Tato, F. et al. Transcatheter Coil Embolization of an Aneurysm of the Pancreatico-duodenal Artery with Occluded Celiac Trunk. Cardiovasc Intervent Radiol 28, 259–261 (2005). https://doi.org/10.1007/s00270-004-0116-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-004-0116-3