Abstract
We report the successful angioplasty of an acute arterial narrowing after suture-mediated closure (SMC) of a femoral arterial puncture. A 75-year-old woman underwent a cerebral arteriogram via a right common femoral artery puncture. The arteriotomy site was closed with a SMC device. Four days after placement the patient complained of pain in her right calf after walking. An arteriogram 7 days after SMC showed a severe focal stenosis at the origin of the superficial femoral artery involving the presumed puncture site. The lesion was successfully treated with balloon angioplasty. The patient at 6 months was asymptomatic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The use of access site closure devices has expanded rapidly in the past several years. Initial reports indicated remarkable safety with these devices; however, a review of the literature reveals a small number of major complications [1 2]. Angio-Seal has the highest rate of major complications (3.2%), followed in descending order by Perclose (2.3%), manual compression (1.0%), and Vasoseal (0.6%) [3 4]. It is important for the interventionalist utilizing these devices to be aware of potential problems, recognize when they occur, and then be able to determine a proper course of treatment.
This case report describes balloon angioplasty in the management of a puncture-site arterial stenosis complicating suture-mediated closure (SMC) of the arterial puncture.
Case Report
A 75-year-old woman underwent a cerebral angiogram from a right common femoral artery puncture for evaluation of transient ischemic attacks. The right common femoral artery was accessed with a #19 gauge single-wall needle and a 5 Fr sheath placed. After the procedure, a femoral arteriogram was performed and the arteriotomy site was closed with a SMC device (Closer, Perclose, Menlo Park, CA, USA) (Fig. 1A) without immediate complication. The evening after the cerebral arteriogram the patient complained of pain in her right groin radiating down her leg. The patient was afebrile and her white blood cell count was normal. Her distal pulses were palpable with no preprocedural change. There was no evidence of a hematoma or bruit at the puncture site. The patient was discharged the day after the arteriogram, and the groin pain resolved 3 days after the procedure.
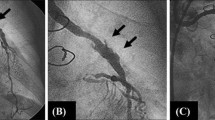
One week later the patient presented with acute right calf claudication (approximately 100 yards) and was re-admitted to the hospital. The right ankle brachial index (ABI) was 0.36 (preprocedural ABI = 0.93). A right lower extremity arteriogram was performed from the left femoral approach, which showed a tight stenosis at the origin of the right superficial femoral artery (Fig. 1B). After discussion with the referring vascular surgeon, a decision was made to treat the lesion with balloon angioplasty. A 5.5 Fr contralateral introducer (Balkin, Cook, Bloomington, IN, USA) was advanced into the right external iliac artery. The proximal superficial femoral artery stenosis was negotiated with a 4 Fr hydrophilic-coated Cobra catheter and a 0.035 inch angled hydrophilic-coated torque wire (Medi-tech, Watertown, MA, USA) using road-mapping. Angioplasty was performed with a 4 mm diameter × 20 mm long RxVIA/TRAC 14 monorail balloon catheter over a 0.014 inch Sparta Core wire (Guidant, Temecula, CA, USA) to 10 atm (147 psi, balloon diameter 4.08 mm at 10 atm). The lesion was further dilated with a 5 mm diameter × 20 mm long RxVIA/TRAC 14 monorail balloon catheter. The waist was eliminated after inflating the balloon to 15 atm (220.5 psi, balloon diameter = 5.37 mm at 15 atm). The final arteriogram revealed no residual stenosis and excellent blood flow in the superficial femoral artery was noted (Fig. 1C).
The following day after the balloon angioplasty, ankle pressure measurement revealed an ABI of 0.88. The patient had no claudication symptoms. The patient has remained asymptomatic for 6 months after angioplasty.
Discussion
Arterial narrowing related to the use of a SMC device is rare. To our knowledge there has been no published report on the use of balloon angioplasty in the management of puncture site stenosis complicating the use of a closure device.
The three commonly used SMC devices are the Closer, Prostar XL, and Prostar plus (Perclose, Menlo Park, CA, USA). The Closer is the current 6 Fr platform, which replaced the Techstar 6 Fr system and is indicated for use in 5 and 6 Fr sheath access sites. The device is inserted over a standard guidewire until pulsatile flow is present through a marker lumen. Footplates are deployed inside the vessel and the device is retracted until flow ceases in the marker lumen. Two needles are then deployed from outside the vessel into the footplates and the suture (3-0 polyester) is captured and pulled through the arterial wall and subsequently through the device. The sutures are then retrieved and a clinch knot is tied using a knot-tying device. The delivery system is removed and the knot is pulled down to the arteriotomy with tension on the rail suture. A knot pusher is then used to push the knot securely against the arterial wall.
The polyester surgical sutures with the Closer system elicit a minimal acute inflammatory reaction in tissues, followed by gradual encapsulation of the suture by fibrous connective tissue [5]. Polyester surgical sutures are not absorbed, nor is any significant change in tensile strength known to occur in vivo.
It is our belief that the inflammatory reaction may have contributed to the stenosis in our patient. The patient did complain of pain at the puncture site, which may have been related to the inflammatory reaction; however, there was no erythema or warmth at the puncture site. Some authors have advocated excluding balloon angioplasty in patients with inflammatory arteritis with serologic evidence of active disease (elevated erythrocyte sedimentation rate or C-reactive protein), although the effect of disease activity on the outcome of angioplasty is unknown [6]. However, Dong et al. [7] reported an 86% clinical success 6 months after renal angioplasty in patients with Takayasu’s arteritis, even though 45% of the patients had an elevated erythrocyte sedimentation rate at the time of dilatation. In our case the patient did respond to balloon angioplasty even though there may have been underlying inflammation at the SMC site.
Thrombus formation at the SMC site may be another potential cause for the stenosis. This could occur from the trauma caused to the endothelium from the puncture or deployment of the SMC device. Furthermore, the suture material acting as a foreign body could also promote thrombus formation.
We were initially reluctant to perform balloon dilatation on the arteriotomy site 1 week after the initial puncture, because of the theoretical risk of rupturing the artery or creating a pseudoaneurysm. However, after further discussion with the vascular surgeon we proceeded with balloon angioplasty. The region was dilated only to 5 mm to avoid potential complications because of the recent arteriotomy. At 6 months there was no evidence of a pseudoaneurysm by clinical examination.
SMC devices were studied in the STAND I and STAND II Trials [8]. STAND I evaluated the 6 Fr Techstar device in 200 patients following catheterization with 5 Fr, 6 Fr, or 6.5 Fr sheaths. Major complications occurred in 2 patients (1%), 1 patient underwent surgical repair for a pseudoaneurysm, and 1 patient developed local site infection requiring outpatient antibiotics. Minor complications occurred in 3 patients (1.5%); 1 patient developed a hematoma >6 cm, and 2 patients developed a small pseudoaneurysm requiring no further therapy. Postprocedural ultrasound studies were obtained in the first 73 patients per protocol: 2 patients had a small pseudoaneurysm as described above, and no patient had intra-arterial thrombus or luminal narrowing at the closure site.
The STAND II Trial was a multicenter study of 515 patients randomized to SMC versus traditional compression. A sheath size ≥8 Fr was used in 57% of patients. Major complications occurred in 2.4% of the suture closure group and 1.1% of the compression group (p = NS). The median time to hemostasis (19 vs 243 min, p < 0.01) and time to ambulation (3.9 vs 14.8 h, p < 0.01) were significantly shorter for SMC.
The Closer European (CE) Trial was a multicenter registry of 200 patients which examined the safety and effectiveness of the Closer 6 Fr SMC system. The study was designed to detect differences in the observed incidence of major complications at 30 days relative to two pre-specified historical control groups of patients undergoing diagnostic procedures. Major complications occurred in no patients. Minor complications occurred in 5 patients (2.5%; 3 patients (1.5%) developed a hematoma > 6 cm, 1 patient (0.5%) developed vessel narrowing at the insertion site which was successful treated by balloon angioplasty, and 1 patient (0.5%) developed an arteriovenous fistula. The results have not been published in a journal, but are available on the Perclose website [9].
In October 1999, the FDA Center for Devices and Radiological Health issued a warning statement and recommendations related to the use of vascular hemostasis devices [10]. Table 1 summarizes the FDA recommendations.
The FDA’s recommendations were followed during deployment of the device in our patient. A single-wall anterior puncture was utilized. The postprocedural management instructions and ambulation recommendations were followed. The patient was not on glycoprotein IIB/IIIA inhibitors or anticoagulated.
It is our belief the anteroposterior (AP) femoral arteriogram before the arterial access closure shows a low puncture of the common femoral artery just above the origins of the superficial and deep femoral arteries (Fig. 1A). The femoral bifurcation is not in profile. An ipsilateral oblique projection would show the true puncture site to be lower than on the AP arteriogram involving the origin of the superficial femoral artery. This additionally likely contributed to the stenosis, since the manufacture recommends puncturing the middle portion of the common femoral artery. Since this complication we have performed an ipsilateral oblique and AP arteriogram on all patients before placing a SMC device. If the puncture site is near the femoral bifurcation we do not use a SMC device.
In summary, stenoses caused by placement of the 6 Fr SMC device can be successfully treated with balloon angioplasty, eliminating the need for surgery. Making sure the arteriotomy site is over the middle of the common femoral artery may limit this complication.
A Right femoral anteroposterior digital subtraction arteriogram (DSA) taken before closure of the puncture site shows a 5 Fr sheath in the common femoral artery immediately proximal to the origins of the deep and superficial femoral arteries. The small arrow indicates the presumed site of the arterial puncture. Note the true femoral bifurcation is not seen in profile. The bifurcation is higher, as shown by the larger arrow. B One week after the procedure a right femoral DSA was performed from a left femoral approach, showing a severe stenosis (arrow) at the origin of the diminutive superficial femoral artery and a moderate narrowing at the origin of the deep femoral artery. C Post-angioplasty arteriogram shows satisfactory dilatation of the stenosis with excellent flow in the superficial femoral artery. The stenosis of the deep femoral artery is again seen.
References
R Wakesman SB King JS Douglas Y Shen H Ewing L Mueller Z Ghazzal WS Weintraub (1995) ArticleTitlePredictors of groin complications after balloon and new device coronary intervention. Am J Cardiol 75 886–889 Occurrence Handle10.1016/S0002-9149(99)80681-X Occurrence Handle7732995
PC Haas Z Krajcer EB Deithrich (1999) ArticleTitleClosure of large percutaneous access sites using the Prostar XL percutaneous vascular surgery device. J Endovasc Surg 6 168–170 Occurrence Handle1:STN:280:DyaK1MvgtVeitg%3D%3D
KL Shrake (2000) ArticleTitleComparison of major complication rates associated with four methods of arterial closure. Am J Cardiol 85 1024–1025 Occurrence Handle1:STN:280:DC%2BD3c3ntlKktQ%3D%3D
MD Gonze WC Sternbergh K Salartash SR Money (1999) ArticleTitleComplications associated with percutaneous closure devices. Am J Surg 178 209–211 Occurrence Handle1:STN:280:DyaK1MvlsVGltw%3D%3D
From the package insert with the Closure device.
JH Park HC Han SH Kim BH Oh YB Park JD Seo (1989) ArticleTitleTakayasu arteritis: Angiographic findings and results of angioplasty. AJR Am J Roentgenol 153 1069–1074
ZJ Dong SH Li XC Lu (1987) ArticleTitlePercutaneous transluminal angioplasty for renovascular hypertension in arteritis: Experience in China. Radiology 162 477–479 Occurrence Handle1:STN:280:BiiD1M3pvFc%3D
DS Baim WD Knopf T Hinohara DE Schwarten RA Schatz CA Pinkerton DE Cutlip M Fitzpatrick KL Ho Kalon RE Kuntz (2000) ArticleTitleSuture-mediated closure of the femoral access site after cardiac catheterization: Results of the Suture To Ambulate and Discharge (STAND I and STAND II) trials. Am J Cardiol 85 864–869 Occurrence Handle1:STN:280:DC%2BD3c3itVKhuw%3D%3D
Available at http://www.perclose.com. Accessed May 23, 2001
JE Henney (1999) ArticleTitleComplications related to vascular hemostasis devices [from the Food and Drug Administration]. JAMA 282 1995 Occurrence Handle1:STN:280:DC%2BD3c%2Flslehuw%3D%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gemmete, J., Dasika, N., Forauer, A. et al. Successful Angioplasty of a Superficial Femoral Artery Stenosis Caused by a Suture-Mediated Closure Device . CVIR 26, 410–412 (2003). https://doi.org/10.1007/s00270-003-2649-2
Issue Date:
DOI: https://doi.org/10.1007/s00270-003-2649-2