Abstract
The stability and equation of state for the cotunnite phase in TiO2 were investigated up to a pressure of about 70 GPa by high-pressure in situ X-ray diffraction measurements using a laser-heated diamond anvil cell. The transition sequence under high pressure was rutile → α-PbO2 phase → baddeleyite phase → OI phase → cotunnite phase with increasing pressure. The cotunnite phase was the most stable phase at pressures from 40 GPa to at least 70 GPa. The equation of state parameters for the cotunnite phase were established on the platinum scale using the volume data at pressures of 37–68 GPa after laser annealing, in which the St value, an indicator of the magnitude of the uniaxial stress component in the samples, indicates that these measurements were performed under quasi-hydrostatic conditions. The third-order Birch-Murnaghan equation of state at K 0′ = 4.25 yields V 0 = 15.14(5) cm3/mol and K 0 = 294(9), and the second-order Birch-Murnaghan equation of state yields V 0 = 15.11(5) cm3/mol and K 0 = 306(9). Therefore, we conclude that the bulk modulus for the cotunnite phase is not comparable to that of diamond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) has many polymorphs, with anatase, brookite, and rutile being the most abundant in nature. The structures and stabilities of the high-pressure phases of TiO2 are of great interest in the geosciences because of the accessible analog of metal dioxide in the rocky mantle and core of the Earth. Under high pressure, TiO2 transforms into (in order of increasing pressure) α-PbO2, baddeleyite, OI, and cotunnite phases (Olsen et al. 1999; Dubrovinskaia et al. 2001, 2002; Dubrovinsky et al. 2001; Ahuja and Dubrovinsky 2002a, b). Recently, Mattesini et al. (2004) reported the existence of a fluorite-like cubic phase together with the cotunnite phase at 48 GPa after heating at 1,900–2,100 K, and proposed that this cubic phase is the densest TiO2 phase above 40 GPa based on the equations of states for both the cubic and cotunnite phases. They also suggested that the cubic phase, rather than the cotunnite phase, is the most stable phase above 40 GPa. Since several metal dioxides are known to transform into the cubic fluorite phase at high pressure, the existence of a fluorite phase has been postulated for TiO2 (Lagarec and Desgreniers 1995; Haines et al. 1993). However, theoretical calculations predict that the cotunnite phase is more stable than the fluorite phase even above 40 GPa (Muscat et al. 2002), and the formation of the cotunnite phase has been reported at 64 GPa after heating at 1,800 K (Dubrovinsky et al. 2001; Dubrovinskaia et al. 2002; Ahuja and Dubrovinsky 2002a, b). Recent experimental and theoretical work also reported the formation of the cotunnite phase at 56–48 GPa and 1,800 K (Al-Khatatbeh et al. 2009). Therefore, the stability of the cotunnite phase in TiO2 still remains controversial.
It is also claimed that cotunnite-type TiO2 is the hardest known oxide as a very high isothermal bulk modulus and microhardness (Dubrovinsky et al. 2001; Dubrovinskaia et al. 2002; Ahuja and Dubrovinsky 2002a, b). The isothermal bulk modulus of the cotunnite phase was reported to be 431 GPa (Dubrovinsky et al. 2001; Dubrovinskaia et al. 2002; Ahuja and Dubrovinsky 2002a, b), and this value is close to that of diamond (444 GPa) (Sung and Sung 1996). However, the experiments in these studies were apparently conducted under non-hydrostatic conditions, because these experiments were performed without a pressure medium and the volume measurements were made by varying the pressure. This procedure produces large stress inhomogeneities in a sample. Moreover, these previous studies failed to mention the pressure scales that were used; this is a serious omission since pressure scales are crucial when discussing equations of state at very high pressures. No other experiments under hydrostatic conditions have been reported, and a recent theoretical study predicts the bulk modulus for the cotunnite phase to be in the range 221–362 GPa (Caravaca et al. 2009; Al-Khatatbeh et al. 2009). Therefore, it is still unclear whether the bulk modulus of the cotunnite phase is similar to that of diamond.
Here, we report the stability and the equation of state for the cotunnite phase in TiO2 up to 70 GPa under quasi-hydrostatic conditions using a diamond anvil cell combined with laser annealing.
Experiment
In our experiments, rutile (99.9%, Soekawa Chemicals) was used as the starting material. It was mixed with a fine platinum powder, which was used as a laser absorber and a pressure calibrant. High pressures were generated using a lever-type diamond anvil cell with 350 μm culet diamonds. The samples were sandwiched between NaCl pellets and loaded into a hole that was drilled into a rhenium gasket. The samples, together with the NaCl pellets, were dried in a drying furnace at 130°C after sampling. NaCl was employed as the pressure-transmitting medium to reduce deviatoric stresses and temperature gradients in the sample chamber. Heating was carried out by irradiating with Nd:YAG laser beams from two sides with a 50–80 μm diameter heating spot.
Angle-dispersive X-ray diffraction (XRD) measurements were conducted at high pressure and room temperature using the BL13A beamline at the Photon Factory of KEK, Japan. This beamline provides a collimated beam (30 μm in diameter) of monochromatic X-ray radiation (wavelength: 0.4266 Å). The XRD spectra were recorded using an imaging plate detector. The sample–detector distance and inclination of the imaging plate were calibrated using a standard material (CeO2) at 1 atm. Two-dimensional XRD images were integrated as a function of 2θ to obtain conventional one-dimensional diffraction profiles.
Pressures were determined from the observed unit-cell volume of platinum using the equation of state proposed by Holmes et al. (1989), and the pressure errors were calculated from uncertainties in the unit-cell volume of platinum. The St value, an indicator of the magnitude of the uniaxial stress component in the samples, was calculated from the (111), (200), and/or (220) diffraction lines of platinum (Shim et al. 2000). The obtained St value was smaller than 0.005, which indicates that the uniaxial component was sufficiently small and that the volume measurements were performed under quasi-hydrostatic conditions (Shim et al. 2000). The experimental conditions, together with the St values, are summarized in Table 1.
Phase relations in TiO2 and stability of the cotunnite phase at high pressure
The first run was conducted in compression up to about 44 GPa; Fig. 1 shows representative XRD patterns obtained in this run. The experimental conditions and the product phases are summarized in Table 1. The starting material was compressed to 11.9 GPa at room temperature. The XRD pattern at 11.9 GPa before heating was broad and contained reflections due to rutile and the baddeleyite phase (Fig. 1a). This is in good agreement with previous studies in which the transition from rutile and anatase to the baddeleyite phase was observed at about 12 GPa without heating (Dubrovinskaia et al. 2001; Haines and Lėger 1993). After heating at 2,200 K for 20 min, the pressure increased to 13.2 GPa at room temperature and the XRD pattern changed to one indicating the coexistence of the α-PbO2 and baddeleyite phases with a minor amount of rutile (Fig. 1b). Then we repeated the XRD observations after heating the sample at 2,500 K at each increment of pressure. The diffraction lines from the α-PbO2 phase became weak and the single phase of baddeleyite was obtained at 19.1 GPa (Fig. 1c). The XRD patterns at pressures of 22.5–28.7 GPa reveal the coexistence of the baddeleyite and OI phases (Fig. 1d). The OI phase was solely stable at 35.3–36.2 GPa (Fig. 1e). The cotunnite phase appeared, accompanied with the OI phase, at 37.4 GPa (Fig. 1f), and the OI phase disappeared at 40.7 GPa. The cotunnite phase was observed even at 43.6 GPa after heating at 2,500 K for 10 min (Fig. 1g). The fluorite-like cubic phase reported by Mattesini et al. (2004) was not observed at all during this run.
Representative XRD patterns (λ = 0.4266 Å) at room temperature during compression during run 1 obtained prior to heating (a), obtained after heating at 2,200–2,500 K (b–g)(see Table 1). A α-PbO2, B NaCl-B2, Bd baddeleyite, C cotunnite, N NaCl-B1, O OI, P platinum, R rutile
In the second run, XRD observations were conducted during compression and decompression with and without heating. Representative XRD patterns obtained during the second run are shown in Fig. 2, and the experimental conditions and the product phases are listed in Table 1. The starting material was compressed to 55 GPa and then heated. After heating at 1,600 K for 60 min, the pressure dropped to 50.7 GPa and the cotunnite phase was observed (Fig. 2a). The sample was further compressed by applying a high pressure and heated at 1,600–2,000 K. Only the cotunnite phase was stable during compression up to 67.6 GPa (Fig. 2b), in agreement with previous studies that have observed the cotunnite phase up to 80 GPa (Dubrovinsky et al. 2001; Dubrovinskaia et al. 2002; Ahuja and Dubrovinsky 2002a, b). On decompression and heating at 2,000 K, the cotunnite phase was stable at 51.1 GPa (Fig. 2c). The sample was further decompressed to 33.9 GPa at room temperature, and the reflections from the cotunnite phase became broad (Fig. 2d). The cotunnite phase was still observed at 18.6 GPa (Fig. 2e). In the recovered sample at ambient conditions, the cotunnite phase disappeared and only weak diffraction peaks from the α-PbO2 phase were observed (Fig. 2f). Once again, the fluorite-like cubic phase was not observed during this compression and decompression process.
Representative XRD patterns (λ = 0.4266 Å) at 300 K during compression and decompression in run 2. (a) 50.7 GPa after heating at 1,600 K, (b) 67.6 GPa after heating at 2,000 K, (c) 51.1 GPa after heating at 2,000 K, (d) 33.9 GPa without heating, (e) 18.6 GPa without heating and (f) ambient conditions. A α-PbO2, B NaCl-B2, C cotunnite, N NaCl-B1, P platinum
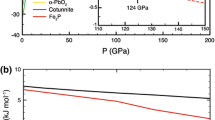
The phase transition sequence with increasing pressure was found to be rutile → α-PbO2 phase → baddeleyite phase → OI phase → cotunnite phase in this study. The cotunnite phase was synthesized from the OI phase at 37.4 GPa after heating at 2,500 K during the compression process in run 1 (Fig. 1). It was also synthesized directly from the starting material (rutile) at 50.7 GPa and remained stable at 67.6 GPa after heating in run 2 (Fig. 2). Our results suggest that the cotunnite phase is the most stable phase at pressures from 37.4 GPa to at least 67.6 GPa, while we could not observe the fluorite-like cubic phase that Mattesini et al. (2004) found at 48 GPa after heating at 1,900–2,100 K, which they proposed as the densest phase at pressures above 40 GPa. We measured the unit-cell volumes of each phase carefully after laser annealing, and the results are plotted in Fig. 3. The unit-cell volumes of the cubic phase reported by Mattesini et al. (2004) are larger than those of the cotunnite phase up to about 60 GPa in this study (Fig. 3). This is consistent with the cubic phase not being observed in this study. The unit-cell volume for the cubic phase becomes smaller than the cotunnite phase observed in this study at pressures above 60 GPa, suggesting that the cubic phase may form at pressures above 60 GPa; however, the cubic phase was not observed above 60 GPa in this study. Theoretical calculations (Muscat et al 2002) predict that the cotunnite phase is more stable than the fluorite phase even at 70 GPa, in good agreement with the results of the present study. Mattesini et al. (2004) discussed the stability of the cubic phase by comparing the equations of state of the cubic and cotunnite phases reported by Dubrovinsky et al. (2001). However, the equation of state for the cotunnite phase reported by Dubrovinsky et al. (2001) is quite different from the cotunnite phase observed in this study (see Fig. 3); this is discussed in detail later below. The unit-cell volume of the cotunnite phase observed in the present study also differs markedly from that reported by Mattesini et al. (2004). Since the experiments of Mattesini et al. (2004) were conducted by directly compressing the powdered sample, the cubic phase reported by Mattesini et al. (2004) could be the metastable phase obtained under non-hydrostatic conditions. Therefore, we can conclude that the cotunnite phase rather than the fluorite-like cubic phase is the most stable phase at pressures from 40 GPa to at least 70 GPa.
Pressure dependence of volume of the high-pressure polymorphs of TiO2 at room temperature. Solid symbols represent the results of the present study obtained after laser annealing, and solid lines represent the compression curves for each phase obtained by fitting the second-order Birch-Murnaghan equation of state (see Table 3). Open symbols represent the cotunnite and fluorite phases in the previous studies (Dubrovinsky et al. 2001; Ahuja and Dubrovinsky 2002a, b; Dubrovinskaia et al. 2002; Mattesini et al. 2004). Dotted and dashed lines represent the compression curves for the cotunnite and fluorite phases in the previous studies, respectively
Equation of state for the cotunnite phase
The unit-cell volumes for the TiO2 polymorphs at room temperature observed in the present study are shown in Fig. 3 together with those reported previously (Dubrovinsky et al. 2001; Dubrovinskaia et al. 2002; Ahuja and Dubrovinsky 2002a, b; Mattesini et al. 2004). The unit-cell parameters of each phase after laser annealing are listed in Table 2. The hydrostaticity of the applied pressure can be checked from the consistency of the d values of a different index, which is expressed by the value of St (Shim et al. 2000). We can conclude that our volume data were obtained under quasi-hydrostatic conditions (Shim et al. 2000), because the St values calculated from the present diffraction data are smaller than 0.005 (Table 1). The volume data for the TiO2 polymorphs were fitted using the third-order Birch-Murnaghan equation of state, as given by
where V 0 is the volume at zero pressure, K 0 is the isothermal bulk modulus, and K 0′ is the first pressure derivative of the isothermal bulk modulus. If we fix K 0′ at 4, we obtain the second-order Birch-Murnaghan equation of state. The fitted results for the TiO2 polymorphs are summarized in Table 3, where they are also compared with previously reported results.
The cotunnite phase is unquenchable under ambient conditions, and thus the fitting parameters are V 0, K 0, and K 0′. However, since the fitting of three parameters produces large uncertainties, we calculated V 0 and K 0 by fixing K 0′. Figure 4 shows the fitted V 0 and K 0 values and the sum of squared residuals (SSR) obtained by using different K 0′ values based on Eq. (1). Very high K 0 values (419–485 GPa) can be obtained if we assume very small K 0′ values (1–2) (Fig. 4), but a large SSR remains in this case. SSR was minimized when K 0′ was fixed as 4.25 (Fig. 4). The third-order Birch-Murnaghan equation of state at K 0′ = 4.25 yields V 0 = 15.14(5) cm3/mol and K 0 = 294(9), while the second-order Birch-Murnaghan equation of state yields V 0 = 15.11(5) cm3/mol and K 0 = 306(9) (Table 3). These results are consistent with those predicted by GGA calculations (V 0 = 15.28 cm3/mol and K 0 = 281 at K 0′ = 4.8) performed by Caravaca et al. (2009) and obtained by experiment (V 0 = 15.22 cm3/mol and K 0 = 312 at K 0′ = 4) reported by Al-Khatatbeh et al. (2009).
The parameters of the equation of state for the cotunnite phase obtained in the present study (V 0 = 15.11–15.14 cm3/mol, K 0 = 294–306 GPa and K 0′ = 4–4.25) are quite different from those reported in previous experimental studies (V 0 = 15.82 cm3/mol, K 0 = 431 GPa, and K 0′ = 1.35) (Dubrovinsky et al. 2001; Dubrovinskaia et al. 2002; Ahuja and Dubrovinsky 2002a, b). Although the amount of numerical data reported in previous studies is quite limited, a few unit-cell volumes for the cotunnite phase at around 50–60 GPa reported in previous studies (Dubrovinsky et al. 2001; Ahuja and Dubrovinsky 2002a, b; Dubrovinskaia et al. 2002; Mattesini et al. 2004) are plotted in Fig. 3. Figure 3 clearly shows that not only the equation of state parameters but also the absolute values of the unit-cell volumes are quite different, and the volumes at high pressure differ by more than 5% from those of the present study. In previous studies, the volume data were collected by varying the pressure at room temperature without using a pressure medium and without annealing. These experimental procedures are likely to generate large stress inhomogeneities in samples. Moreover, the earlier studies do not mention anything about the pressure scales used. Although it is difficult to believe that the stress state and the pressure scale alone are responsible for such a large discrepancy in the unit-cell volume, further analysis is impossible because of the lack of detailed information in the previous studies (Dubrovinsky et al. 2001; Dubrovinskaia et al. 2002; Ahuja and Dubrovinsky 2002a, b; Mattesini et al. 2004). On the other hand, we carefully annealed the sample and checked the stress state by calculating the St value, which indicates that our X-ray measurements were performed under quasi-hydrostatic conditions. Therefore, we can say that the measurements in the present study are more reliable and that the isothermal bulk modulus, K 0, for the cotunnite phase lies within the range of about 294–306 GPa. These values are significantly smaller than that of diamond (444 GPa) (Sung and Sung 1996), and thus we conclude that the bulk modulus for the cotunnite phase of TiO2 is not comparable to that of diamond.
References
Ahuja R, Dubrovinsky LS (2002a) Cotunnite-structured titanium dioxide and the hardest known oxide. High Press Res 22:429–433. doi:10.1080/08957950212787
Ahuja R, Dubrovinsky LS (2002b) High-pressure structural phase transition in TiO2 and synthesis of the hardest know oxide. J Phys Condens Matter 14:10995–10999
Al-Khatatbeh Y, Lee KKM, Kiefer B (2009) High-pressure behavior of TiO2 as determined by experiment and theory. Phys Rev B 79:134111
Arlt T, Bermejo M, Blanco MA, Gerward L, Jiang JZ, Olsen JS, Recio JM (2000) High-pressure polymorphs of anatase TiO2. Phys Rev B 61:14414–14419
Caravaca MA, Miño JC, Pérez VJ, Casali RA, Ponce CA (2009) Ab intio study of the elastic properties of single and polycrystal TiO2, ZrO2 and HfO2 in the cotunnite structure. J Phys Condens Matter 21:015501. doi:10.1088/0953-8984/21/1/015501
Decker DL (1971) High-pressure equation of state for NaCl, KCl, and CsCl. J Appl Phys 42:3239–3244
Dubrovinskaia NA, Dubrovinsky LS, Ahuja R, Prokopenko VB, Dmitriev V, Weber HP, Osorio-Guillen JM, Johansson B (2001) Experimental and theoretical identification of a new high-pressure TiO2 polymorph. Phys Rev Lett 87:27550. doi:10.1103/PhysRevLett.87.275501
Dubrovinskaia NA, Dubrovinsky LS, Swamy V, Ahuja R (2002) Cotunnite-structured titanium dioxide. High Press Res 22:391–394. doi:10.1080/08957950212781
Dubrovinsky LS, Dubrovinskaia NA, Swamy V, Muscat J, Harrison NM, Ahuja R, Holm B, Johansson B (2001) The hardest known oxide. Nature 410:653–654
Gerward L, Olsen LS (1997) Post-rutile high-pressure phase in TiO2. J Appl Cryst 30:259–264
Haines J, Lėger M (1993) X-ray diffraction study of TiO2 up to 49 GPa. Physica B 192:223–237
Haines J, Lėger JM, Chateau C (1993) X-ray diffraction study of the phase transitions and structural evolution of tin dioxide at high pressure: relationships between structure types and implications for other rutile-type dioxides. Phys Rev B 55:11144–11154
Heinz DL, Jeanloz R (1984) Compression of the B2 high-pressure phase of NaCl. Phys Rev B 30:6045–6050
Holmes NC, Moriarty JA, Gathers GR, Nellis WJ (1989) The equation of state of platinum to 660 GPa (6.6 Mbar). J Appl Phys 66:2962–2967
Lagarec K, Desgreniers S (1995) Raman study of single crystal anatase TiO2 up to 70 GPa. Solid State Commun 94:519–524
Mao HK, Bell PM, Shaner JW, Steinberg DJ (1978) Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R 1 fluorescence pressure gauge from 0.06 to 1 Mbar. J Appl Phys 49:3276–3283
Mattesini M, de Almeida JS, Dubrovinsky L, Dubrovinskaia N, Johansson B, Ahuja R (2004) High-pressure and high-temperature synthesis of the cubic TiO2 polymorph. Phys Rev B 70:212101. doi:10.1103/PhysRevB.70.212101
Muscat J, Swamy V, Harrison NM (2002) First-principles calculations of the phase stability of TiO2. Phys Rev B 65:224112. doi:10.1103/PhysRevB.65.224112
Olsen S, Gerward L, Jiang JD (1999) On the rutile/α-PbO2-type phase boundary of TiO2. J Phys Chem Solids 60:229–233
Sato-Sorensen Y (1983) Phase-transitions and equations of state for the sodium-halides: NaF, NaCl, NaBr, and NaI. J Geophys Res 88:3543–3548
Shim SH, Duffy TS, Shen G (2000) The equation of state of CaSiO3 perovskite to 108 GPa at 300 K. Phys Earth Planet Inter 120:327–338
Sung CM, Sung M (1996) Carbon nitride and other speculative superhard materials. Mater Chem Phys 43:1–18
Swamy V, Dubrovinskaia NA, Dubrovinsky LS (2002) Compressibility of baddeleyite-type TiO2 from static compression to 40 GPa. J Alloy Compd 340:46–48
Acknowledgments
High-pressure in situ X-ray experiments were conducted at the Photon Factory under the auspices of proposal 08G042. We are grateful to the anonymous referees and the Editor for their constructive reviews. D. N–H is supported by a Research Fellowship from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishio-Hamane, D., Shimizu, A., Nakahira, R. et al. The stability and equation of state for the cotunnite phase of TiO2 up to 70 GPa. Phys Chem Minerals 37, 129–136 (2010). https://doi.org/10.1007/s00269-009-0316-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-009-0316-0