Abstract
Radiation effects on kaolinite were investigated using He+ ions of 1.5 MeV at radiation doses up to 4.3 × 108 Gy, which are comparable to the doses expected for clay barriers in high-level nuclear waste repositories. The concentration of paramagnetic radiation-induced defects in kaolinite reaches 2 × 1016 spins/mg (400 at. ppm), as determined by electron paramagnetic resonance spectroscopy. The broadening of X-ray diffraction patterns and transmission infrared (IR) absorption bands is mostly related to the structural strain induced by radiation-induced point defects. The broadening of IR absorption spectra is analyzed using an autocorrelation approach and is related to a change in the distribution of vibrational frequencies due to crystal heterogeneities. We theoretically analyze how the effective dielectric properties of kaolinite samples depend on macroscopic parameters and how irradiation can modify some of them. Irradiation leads to an increase in the electronic polarizability of kaolinite particles, related to the accumulation of radiation-induced electronic point defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clay minerals are important materials for the disposal of high-level nuclear wastes (HLNWR) in geologic environments. They can occur in the host rocks or be used in the engineered barriers used for radioactive waste confinement. Owing to their reactivity and large specific surface area, they are used to limit the leaching and dissemination of radionuclides in the environment. A safety analysis requires the understanding of the effects of intense irradiation, not only on waste forms (Ewing et al. 1995; Delaye and Ghaleb. 2000), but also in the surrounding clay matrix (Allard and Calas, 2008). Change of atomic scale properties by ionizing radiations, concerning, e.g., electronic defects or redox states, have received much attention, as ionizing radiations interact with electrons, whereas the amount of displaced atoms is small. The irradiation of kaolin-group minerals and montmorillonite by various types of radiation (α, β, γ rays) and doses result in the formation of various defect centers and a modification of Fe-oxidation state (Allard et al. 1994; Pushkareva et al. 2002; Allard and Muller 1998; Sorieul et al. 2005; Gournis et al. 2000, 2001; Ploetze et al. 2003). Radiation dosimetry based on clay minerals provides information on past radionuclide transfers in natural environments (Allard and Muller 1998; Allard et al. 2003, 2007) and is an original way of dating soil-forming processes (Balan et al. 2005a). The various aspects of irradiation effects on clay minerals with emphasis on physico-chemical properties have been reviewed recently (Allard and Calas 2008). Because the oxidation state of structural iron in smectite influences the charge of the layer, the effect of ionizing radiation may have important consequences upon clay properties such as CEC, hydration and swelling. Besides, it was shown that heavy ions such as alpha recoil nuclei may lead to amorphization of smectite over a duration consistent with HLNWR (Sorieul et al. 2008), a process that may alter swelling, water retention, cation exchange capacity and solubility.
This study deals with the effects of 1.5 MeV He+ ions on well-ordered kaolinite. The studied sample is a hydrothermal kaolinite DCV with a very high cristallinity (Gaite et al. 1997; Clozel et al. 1995; Balan et al. 1999, 2000). The ionizing irradiation has been performed in thin films, in which ballistic effects have been avoided. Increasing defect concentrations up to 1.3 × 1016 spins/mg leads to broadening of X-ray diffraction profiles, due to heterogeneous strain, as particle-size effect is not significant. Infrared spectroscopy indicates the influence of a high concentration of electronic point defects, which leads to an increase in the electronic polarizability of kaolinite and hence of the refractive index.
Materials and methods
The DCV kaolinite sample is an exceptionally well-ordered hydrothermal kaolinite from Decazeville (France). The surface area is c.a. 1 m2 g−1 and the particles (Fig. 1) are thicker that the ones of well-ordered KGa-2 kaolinite (Zbik and Smart 1998). This high crystallinity is important to study well-resolved bands in infrared spectra. The aspect ratio of the particles of DCV kaolinite obtained from AFM observations is a/c = 0.156 (Sayed Hassan et al. 2005). This exceptional sample is ideal to investigate the faint structural changes due to high doses of ionizing radiations by minimizing the influence of stacking faults and other structural defects inherent to less ordered kaolinite.
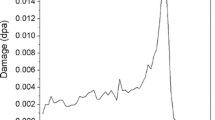
The clayey fraction (Φ < 5 μm) was separated by sedimentation. Suspensions were obtained by adding few drops of 0.1 M NaOH up to a pH of 7.5. About 100 mg of kaolinite was deposited on plates of 10 cm diameter by sedimentation to form a thin film of 4 μm ensuring that helium ions are not implanted in the kaolinite sample (Fig. 2). The energy losses predominantly correspond to ionization processes (more than 99%) and the error on the deposited dose was estimated at ±10%. Default values of the SRIM code (Ziegler et al. 1998) were accepted for displacement energy (20 eV). For this sample thickness, implantation of He atoms in particles is minimized (Fig. 2).
Energy loss by ionization and He implantation with the depth penetration in the sample for 1.5 MeV alpha particles in kaolinite calculated with the TRIM code (Ziegler et al. 1998)
Irradiation experiments were performed with a He+ beam at high fluence using the Van De Graff accelerator of CSNSM (Orsay, France). The beam energy and current were 1.5 MeV and 5–15 μA (depending on the irradiation dose), respectively. The irradiation experiments last from 4 min (21 MGy) to approximately one hour for the highest dose (427 MGy). The dose rate are kept below 250 kGy s−1 to avoid possible recombination of the paramagnetic defects with an increase of temperature. Unstable electronic defects are usually produced during artificial irradiation, and thermally recombine as a function of time following exponential decay laws. The rapid initial decay of these defects makes it difficult to compare different measurements performed just after irradiation but at different times. In order to address only stable defects, we investigated samples stored at room temperature about one year after irradiation.
Powder X-ray patterns of samples deposited on Si holders were recorded using a Philips PW3050/60 diffractometer with Co Kα tube operating at 40 kV and 40 mA. Room temperature X-band EPR spectra were collected at ≈9.4 GHz with a Bruker ESP300E using a modulation amplitude of 3.19 G, a modulation frequency of 100 KHz, and a microwave power of 40 mW. Quantitative measurements were performed using calibrated Suprasil® grade silica tubes and a constant filling factor of the cavity. Total spin concentration was determined by comparing the recorded intensity with a Bruker standard sample (weak pitch). Transmission IR spectra were performed with a Nicolet Magna 560 FT-IR spectrometer at room temperature, between 400 and 4,000 cm−1, with a resolution of 1 cm−1. Pellets for transmission measurements were obtained by pressing a mixture of about 1 mg of kaolinite diluted in 300 mg of dried KBr.
Results and discussion
EPR dosimetry of radiation-induced defects
The EPR spectra of irradiated DCV kaolinite show an intense signal at g = 2 (i.e., 0.336 T), the intensity of which increases with the radiation dose (Fig. 3). Previous studies have shown that this signal corresponds to the superimposition of different defects (Clozel et al. 1995; Allard et al. 1994). The irradiated samples present a relative concentration of Al–O–Al defects (B-centers) higher than that of the pristine sample. No attempt was made to separate the contribution of the different defect centers, and the dosimetry of the irradiated samples was made on the total amount of radiation-induced defects. The defect concentration was therefore calculated by integrating twice the normalized EPR spectra, which correspond to the derivative of sample absorbance (see e.g., Calas 1988). The value is then ratioed to the weak pitch integrated absorbance to obtain a defect concentration expressed in spin/mg. The measured concentration varies from 1.1 × 1014 spin/mg for the pristine sample to 1.87 × 1016 spin/mg for the most irradiated sample (427 MGy). The dosimetry curve, i.e., the evolution of defect concentration versus irradiation dose, is similar to that observed during previous studies on kaolinite (Allard and Muller 1998) and suggests that the production of stable defects saturates at high dose. As only paramagnetic defects with long relaxation times are detected using EPR spectroscopy, the defect concentration provided by EPR measurements should be taken as a minimum value of total defect concentration in irradiated kaolinite.
X-ray diffraction
The XRD patterns of the pristine and irradiated kaolinite do not show any modification of unit-cell parameters as a result of defect accumulation. This is consistent with the observation that swelling due to localized defects is more limited than the swelling in presence of recoil damage (Salje et al. 1999). In addition, the weak hydrogen bonds bridging the layered structure of kaolinite may also absorb a radiation-induced swelling. As the strain-size analysis by Rietveld refinement of clay minerals patterns is difficult to perform, we used a single line approach to determine change in the mean coherent domain (MCD) size and structural strain as a function of radiation dose (Amigo et al. 1994). The full width at half maximum (FWHM) and integral breadth of the (0 0 2) line after the removal of the Kα2 contribution were obtained by fitting the line profile with a split pseudo-Voigt. Other reflexions of the patterns were not used due to overlapping peaks or a weak intensity. The first peak in the [0 0 1] direction is well defined but the Lorentz-polarization factor is strongly decreasing at this scattering angle (Eberl et al. 1996). The size and strain parameters were then obtained using the approach described in Keijser et al. (1982). The results show that FWHM and integral breadth increase with defect concentration (Table 1; Fig 4). However, the variations of MCD size along the [0 0 l] direction are not systematic and this increase is rather related to strain broadening. The local strain values in kaolinite are similar to those observed in weakly irradiated zircon (Salje et al.1999) or titanite (Chrosch et al. 1998) and they increase as defect concentration with radiation dose (Fig. 3).
Modification of IR spectra during irradiation
The IR spectrum of the pristine DCV sample displays intense and well-resolved absorption bands (Fig. 5a, b). At high frequency (3,500–3,800 cm−1), the four bands correspond to stretching vibration of OH groups (Farmer 1998, 2000). The bands observed between 1,000 and 1,200 cm−1 correspond to Si–O stretching modes. The two intense bands at 1,008 and 1,031 cm−1 and the band at 1,115 cm−1 correspond to in-plane vibrations of Si–O bonds. The broader band at 1,103 cm−1 corresponds to the in-phase out-of-plane motion of Si–O bonds and is also significantly affected by the depolarization field perpendicular to the basal plane of kaolinite particles (Balan et al. 2001). The bands observed around 900 cm−1 correspond to bending modes involving OH groups whereas those at lower frequencies involve the coupled motion of various bond types.
The overall shape of the IR spectrum remains similar after irradiation, indicating that no major structural change occurs. However, two types of modifications are observed as a function of the radiation dose (Fig. 5). The first one is an overall broadening of Si–O (Fig. 5b) and OH stretching bands at 3,700 cm−1 (Fig. 5a). This observation is consistent with the broadening under irradiation of zircon micro-Raman spectra (Palenik et al. 2003). Additional changes were observed after the spectrum of pristine kaolinite was convoluted with a Gaussian function to account for broadening effects (Fig. 6a). In particular, a lowering of c.a. 10 cm−1 is observed for the broad Si–O stretching band corresponding to out-of-plane vibrations at c.a. 1,100 cm−1 (Fig. 5). A smaller shift is observed on the band at c.a. 700 cm−1. We will focus on the domain including the Si–O stretching modes and O–H bending modes, i.e., between 850 and 1,350 cm−1. The O–H stretching bands, although sensitive to structural changes, are not considered because of the additional uncertainty related to the subtraction of the baseline due to non structural water.
a Comparison of the 21.35 MGy irradiated DCV spectrum in 400–1,200 cm−1 range with the convoluted spectrum of the pristine sample. Arrow indicates the location of the shift. b Comparison of the 21.35 MGy irradiated sample with the simulated effect of a change of the dielectric constant ε∞ of kaolinite, in a KBr medium. For the dielectric constant, the changes were made on the average value of the electronic dielectric tensor of kaolinite (ε∞ from 2.5 to 10) in a KBr medium (εmed = 2.25)
Origin of radiation-induced broadening of IR spectra
The line profile of high-frequency vibrational modes is usually the convolution of an intrinsic line profile, depending on the damping coefficient of the corresponding harmonic oscillator, with a distribution function of phonon frequencies related to the system heterogeneity (Salje et al. 2000). The accumulation of radiation-induced defects can lead to a broadening of IR lines through a modification of the phonon coupling mechanisms responsible for phonon lifetime and intrinsic line width or through heterogeneous changes in crystal structure, leading to a distribution of vibrational frequencies. Although both types of mechanisms cannot be easily distinguished, the IR linewidth is a useful probe of crystal order. The broadening of complex IR spectra can be efficiently assessed using an autocorrelation approach initially developed to investigate phase transitions in minerals with hard mode spectroscopy (Salje et al. 2000). It is particularly suited to investigate the transformations of low-symmetry minerals such as kaolinite, whose spectrum displays a large number of overlapping bands. The self-convolution of the IR spectrum with an offset ω′ in the frequency leads to the autocorrelation function:
where α(ω) is the spectrum after baseline subtraction in absorbance units. The main information on the line width of the IR spectrum is then obtained from the shape of the autocorrelation function in the limit ω′ → 0. Practically, the method implies to choose some segments of the IR spectra in such a way that the intensity continuously falls to zero on the limits of the segments, i.e., α(ω) = 0 beyond these limits. As expected, the empirical broadening factor obtained from the spectra autocorrelation increases with the dose and defect concentration. Interestingly, the correlation with the defect concentration determined by EPR is not linear. The width parameter displays a quadratic trend as a function of paramagnetic defect concentration (Fig. 7). The probability of phonon scattering by defects is proportional to defect concentration (e.g., Surovtsev et al. 1999). The observed quadratic trend thus suggests that the line broadening in irradiated samples is related to a change in the distribution of vibrational frequencies due to crystal heterogeneities. This interpretation is consistent with the systematic increase of structural strain as a function of dose, as observed using XRD.
Origin of specific band shifts in IR spectra
As compared to IR spectra of bulk materials, powder IR spectra exhibit additional broadening and blue-shift of specific bands (e.g., Farmer 1974; Fuchs 1975; Serna et al. 1987; Salje and Bismayer 1997; Balan et al. 2008; Blanchard et al. 2008). The atomic displacements in a powdered ionic crystal are indeed sensitive to the oscillating macroscopic electric field (depolarization field) induced by the polarization of the particles. This field depends on the particle shape and dielectric properties of crystals and can be computed using the classical laws of electrostatics. It shifts the bands toward higher frequencies and causes band broadening in samples with distributed or non-ellipsoidal particle shapes. An efficient approach has been developed to model the powder absorption spectra of minerals from first-principles (Balan et al. 2001, 2005b, 2007). This approach combines the electrostatic modeling of IR absorption by small ellipsoidal particles embedded in a dielectric matrix and the theoretical computation of the harmonic low-frequency dielectric tensor of the bulk material in the framework of Density Functional Theory. In practice, the absorption spectrum is obtained by calculating the electromagnetic power dissipated in the particle by the internal oscillating electric field and averaged over time and spatial orientation. For small ellipsoidal particles, the internal electric field is homogenous and can be obtained as a function of the dielectric tensor of the particle and external medium using classical boundary conditions. A great advantage of first-principles methods is to provide reliable values of the dielectric tensor of materials for which it can be hardly determined from experiment, such as clay minerals.
Influence of the particle shape
The change in the particle geometry, from plates perpendicular to the c* axis to spherical particles, induces a significant shift of the absorption bands (Fig. 8). In the platy particles, only the bands corresponding to modes mostly polarized along the c* axis are displaced at a frequency higher than that computed for a zero macroscopic electric field (transverse optical mode frequency). A larger number of absorption bands are affected in spherical particles. Interestingly, the OH bending bands at c.a. 900 cm−1 are weakly affected by depolarization effects for these particle shapes.
The comparison of the theoretical kaolinite spectra with that of the pristine DCV sample shows that a ratio of the principal axis of ellipsoidal particles of c.a. 0.2, i.e., oblate ellipsoids, accounts for the relative position of Si–O stretching bands. This ratio is consistent with the value of 0.16 obtained from AFM observations. Here, we remark that the difference between the spectra of the DCV sample and that of the CMS standard sample KGa-1 (Balan et al. 2001) can be explained by the thinner shape of the particles occurring in the latter (Zbik and Smart 1998). For platy particles, the depolarization field shifts the bands polarized along the direction perpendicular to the basal plane of particles toward higher frequencies. The opposite changes observed in the IR spectra of irradiated samples, together with the absence of major changes in the particle shape as observed by SEM, thus indicates that exfoliation of kaolinite particles did not occur during the irradiation experiments. Consistently, XRD patterns do not reveal a systematic decrease in the MCD size along the [0 0 l] direction.
Influence of the electronic polarizability
The powder IR absorption spectra depends on the dielectric properties of the whole sample, which can be considered in the dilute limit as constituted of small clay particles inserted in a homogeneous dielectric matrix, usually KBr. The relations between the dielectric constant of the diluting matrix and the IR spectrum of platy particles of kaolinite have been previously investigated (Balan et al. 2007). The absorbance of the bands affected by depolarization effects increases when the dielectric constant of the external medium increases. In the present case, all spectra have been recorded using a KBr matrix and the observed changes cannot be ascribed to variations of the external medium. In contrast, the presence of point defects in the structure of kaolinite can modify its electronic polarizability. The modification of refractive index of transparent crystals by He+ implantation is a well-known effect which has been investigated for the conception of optical waveguides (Can and Towsend 1994).
The theoretical electronic dielectric tensor of pristine kaolinite is slightly anisotropic with an average value of 2.5 (Balan et al. 2001). Theoretical IR spectra of kaolinite were thus computed for oblate ellipsoidal particles with an aspect ratio of 0.2, using an isotropic electronic dielectric tensor ε(∞) varying between 2.5 and 6 (Fig. 6b). The increase in electronic polarizability induces a red-shift of the bands affected by the depolarization field. The similar evolution observed for the theoretical and experimental spectra suggests that the specific radiation effects observed on the IR spectroscopic properties of kaolinite are related to an increase in its electronic polarizability. Based on the shift observed for the Si–O stretching band, the theoretical increase of the dielectric constant is c.a. 0.3 for a dose of 4.2 × 108 Gy. Shifts of dielectric constant were already observed in different materials at several frequencies (Bayly and Townsend 1973; Katenkamp et al. 1980). For example, an increase of 6% of the refractive index is observed (Webb and Townsend 1976) in silica under N+ implantation up to a fluence of 2 × 1016 ions cm−2. The increase in electronic polarizability observed in the IR range is most likely related to the low-frequency tail of the high-frequency resonances of the dielectric tensor, occurring in the UV region of the electromagnetic spectrum and corresponding to light absorption by electronic defects. FTIR spectroscopy thus offers an alternative way to determine the variations of the refraction index in finely divided materials, which cannot be investigated using standard optical methods.
Concluding remarks
We have investigated the changes in IR spectroscopic properties of kaolinite samples irradiated at high doses of alpha radiations. Although amorphization does not occur, broadening of XRD lines and IR absorption bands as a function of dose suggests that significant distortions of the crystal structure are induced by the accumulation of point defects. Beside, shifts of specific bands indicate that electronic point defects increase the high-frequency electronic polarizability of kaolinite. Therefore, these observations suggest that high alpha-doses of radiation can significantly modify some macroscopic properties of kaolinite samples. In case of kaolinite, the studied physical properties have no immediate influence on the safety of a HLNWR. Nevertheless, kaolinite was investigated as a model of clay in this study. Further investigations are currently performed on smectite, which is a clay proposed as a component of the engineering barriers. In the context of high level radioactive waste disposal, it is necessary to study both ionizing and ballistic irradiation effects and focus on possible radiation-induced reactivity changes.
References
Allard T, Calas G (2008) Influence of radiation effects on clay mineral properties. Appl Clay Sci (accepted). doi:10.1016/j.clay.2008.07.032
Allard T, Muller JP (1998) Kaolinite as an in situ dosimeter for past radionuclide migration at the Earth’s surface. Appl Geochem 13(6):751–765. doi:10.1016/S0883-2927(98)00011-0
Allard T, Muller JP, Dran JC, Menager MT (1994) Radiation-induced paramagnetic defects in natural Kaolinites—alpha-dosimetry with ion-beam irradiation. Phys Chem Miner 21(1–2):85–96. doi:10.1007/BF00205219
Allard T, Ildefonse P, Perez del Villar L, Sorieul S, Pelayo M, Boizot B, Balan E, Calas G (2003) Radiation-induced defects in dickites from the El Berrocal granitic system (Spain): relation with past occurrence of natural radioelements. Eur J Mineral 15(4):629–640. doi:10.1127/0935-1221/2003/0015-0629
Allard T, Calas G, Ildefonse P (2007) Reconstruction of past uranium migration in a sedimentary deposit (Coutras, France): implications for a radwaste repository. Chem Geol 239(1–2):50–63. doi:10.1016/j.chemgeo.2006.12.007
Amigo JM, Bastida J, Sanz A, Signes M, Serrano J (1994) Cristallynity of lower Cretaceous kaolinites of Teruel (Spain). Appl Clay Sci 9:51–69. doi:10.1016/0169-1317(94)90014-0
Balan E, Allard T, Morin G, Hernandez G, Labbe JC, Muller JP (1999) Structural Fe3+ in natural kaolinites: New insights from electron paramagnetic resonance spectra fitting at X and Q band frequencies. Clays Clay Miner 45(5):605–616. doi:10.1346/CCMN.1999.0470507
Balan E, Allard T, Boizot B, Morin G, Muller JP (2000) Quantitative measurement of paramagnetic Fe3+ in kaolinite. Clays Clay Miner 48(4):439–445. doi:10.1346/CCMN.2000.0480404
Balan E, Saitta AM, Mauri F, Calas G (2001) First-principles modeling of the infrared spectrum of kaolinite. Am Mineral 86(11–12):1321–1330
Balan E, Allard T, Fritsch E, Selo M, Falgueres C, Chabaux F, Pierret MC, Calas G (2005a) Formation and evolution of lateritic profiles in the middle Amazon basin: Insights from radiation-induced defects in kaolinite. Geochim Cosmochim Acta 69(9):2193–2204. doi:10.1016/j.gca.2004.10.028
Balan E, Lazzeri M, Saitta AM, Allard T, Fuchs Y, Mauri F (2005b) First-principles study of OH-stretching modes in kaolinite, dickite, and nacrite. Am Mineral 90(1):50–60. doi:10.2138/am.2005.1675
Balan E, Lazzeri M, Mauri F, Calas G (2007) Structure, reactivity and spectroscopic properties of minerals from lateritic soils: insights from ab initio calculations. Eur J Soil Sci 58(4):870–881. doi:10.1111/j.1365-2389.2007.00937.x
Balan E, Blanchard M, Hochepied JF, Lazzeri M (2008) Surface modes of the infrared spectra of hydrous minerals: the OH stretching modes of bayerite. Phys Chem Miner 35:279–285. doi:10.1007/s00269-008-0221-y
Bayly AR, Townsend PD (1973) Ellipsometric analysis of refractive index profiles produced by ion implantation in silica glass. J Applied Phys D Appl Phys (Berl) 6:1115–1128
Blanchard M, Lazzeri M, Mauri F, Balan E (2008) First-principles calculation of the infrared spectrum of hematite. Am Mineral (in press)
Calas G (1988) Electron paramagnetic resonance. Rev Mineral 18:513–571
Can N, Townsend PD (1994) Anomalous annealing of zircon optical waveguides formed by implantation of helium ions. Radiat Eff Defects Solids 128(3):215–220. doi:10.1080/10420159408219760
Chrosch J, Colombo M, Malcherek T, Salje EKH, Groat LA, Bismayer U (1998) Thermal annealing of radiation damage titanite. Am Mineral 83:1083–1091
Clozel B, Gaite JM, Muller JP (1995) Al–O–Al paramagnetic defects in Kaolinite. Phys Chem Miner 22(6):351–356. doi:10.1007/BF00213331
Delaye JM, Ghaleb D (2000) Dynamic processes during displacement cascades in oxide glasses: A molecular-dynamics study. Phys Rev B 61(21):14481–14494. doi:10.1103/PhysRevB.61.14481
Eberl DD, Dritis VA, Irondon J, Nuesch R (1996). Mudmaster: a program for calculating crystallite size distributions and strain from the shapes of X-ray diffraction peaks. US Geological Survey Open File Report 96–171
Ewing RC, Weber WJ, Clinard FW Jr (1995) Radiation effects in nuclear waste forms for high-level radioactive waste. Prog Nucl Energy 29(2):63–127. doi:10.1016/0149-1970(94)00016-Y
Farmer VC (1974) The IR spectra of minerals. Mineral Society, London, pp 331–363
Farmer VC (1998) Differing effects of particle size and shape in the infrared and Raman spectra of kaolinite. Clay Miner 33(4):601–604
Farmer VC (2000) Transverse and longitudinal crystal modes associated with OH stretching vibrations in single crystals of kaolinite and dickite. Spectrochim Acta A Mol Biomol Spectrosc 56(5):927–930. doi:10.1016/S1386-1425(99)00182-1
Fuchs R (1975) Theory of he optical properties of ionic crystal cubes. Phys Rev B 11:1732–1740. doi:10.1103/PhysRevB.11.1732
Gaite JM, Ermakoff P, Allard T, Muller JP (1997) Paramagnetic Fe3+: a sensitive probe for disorder in kaolinite. Clays Clay Miner 45(4):496–505. doi:10.1346/CCMN.1997.0450402
Gournis D, Mantaka-Marketou AE, Karakassides MA, Petridis D (2000) Effect of gamma-irradiation on clays and organoclays: a Mossbauer and XRD study. Phys Chem Miner 27(7):514–521. doi:10.1007/s002690000089
Gournis D, Mantaka-Marketou AE, Karakassides MA, Petridis D (2001) Ionizing radiation-induced detects in smectite clays. Phys Chem Miner 28(4):285–290. doi:10.1007/s002690100153
Katenkamp U, Karge H, Prager R (1980) Radiation defects and optical properties of ion implanted silicon dioxide. Radiat Eff Defects Solids 48(1):31–34. doi:10.1080/00337578008209224
de Keijser Th H, Langford JI, Mittemeijer EJ, Vogels APB (1982) Use of the Voigt function in a single-line method for the analysis of X-ray diffraction line broadening. J Appl Cryst 15:308–314. doi:10.1107/S0021889882012035
Palenik CS, Nasdala L, Ewing RC (2003) Radiation damage in zircon. Am Mineral 88:770–781
Ploetze M, Kahr G, Stengele Hermanns R (2003) Alteration of clay minerals—gamma-irradiation effects on physicochemical properties. Appl Clay Sci 23(1–4):195–202. doi:10.1016/S0169-1317(03)00103-0
Pushkareva R, Kalinichenko E, Lytovchenko A, Pushkarev A, Kadochnikov V, Plastynina M (2002) Irradiation effect on physico-chemical properties of clay minerals. Appl Clay Sci 21(1–2):117–123. doi:10.1016/S0169-1317(01)00097-7
Salje EKH, Bismayer U (1997) Hard mode spectroscopy: the concept and applications. Phase Transit 63:1–75. doi:10.1080/01411599708228789
Salje EKH, Chrosch J, Ewing RC (1999) Is “metamictization” of zircon a phase transition? Am Mineral 84:1107–1116
Salje EKH, Carpenter MA, Malcherek T, Boffa Balaran T (2000) Autocorrelation analysis of infrared spectra from minerals. Eur J Mineral 12(3):503–519
Sayed Hassan M, Villeras F, Razafitianamaharavo A, Michot JL (2005) Role of exchangeable cations on geometrical and energetic surface heterogeneity of kaolinites. Langmuir 21(26):12283–12289. doi:10.1021/la051993n
Serna CJ, Ocana M, Iglesias JE (1987) Optical properties of α-Fe2O3 microcrystals in the infrared. J Phys C Solid State Phys 20:473–484. doi:10.1088/0022-3719/20/3/017
Sorieul S, Allard T, Morin G, Boizot B, Calas G (2005) Native and artificial radiation-induced defects in montmorillonite. An EPR study. Phys Chem Miner 32(1):1–7. doi:10.1007/s00269-004-0427-6
Sorieul S, Allard T, Wang LM, Grambin-Lapeyre C, Lian J, Calas G, Ewing RC (2008) Radiation-stability of smectite. Environ Sci Technol (accepted). doi:10.1021/es800766b
Surovtsev NV, Kupriyanov IN, Malinovsky VK, Gusev VA, Pal’yanov YN (1999) Effect of nitrogen impurities on the Raman line width in diamonds. J Phys Condense Matter 11:4767–4774. doi:10.1088/0953-8984/11/24/316
Webb AP, Townsend PD (1976) Refractive index profiles induced by ion implantation into silica. J Phys D Appl Phys 9:1343–1354. doi:10.1088/0022-3727/9/9/011
Zbik M, Smart RSC (1998) Nanomorphology of kaolinite: comparative SEM and AFM studies. Clays Clay Miner 46(2):153–160. doi:10.1346/CCMN.1998.0460205
Ziegler JF, Biersack JP, Littmark U (1998) The stopping and range of ions in solids. Pergamon, New York
Acknowledgments
We thank Satoshi Utsunomiya and an anonymous reviewer for their constructive reviews. The dielectric tensor of kaolinite was calculated at the IDRIS institute (Institut du Développement et des Ressources en Informatique Scientifique) of CNRS (Centre National de la Recherche Scientifique) within the project 060411519. This work has been supported by the French National Research Agency (ANR, projet “SPIRSE”). This work is IPGP contribution #2420.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fourdrin, C., Balan, E., Allard, T. et al. Induced modifications of kaolinite under ionizing radiation: an infrared spectroscopic study. Phys Chem Minerals 36, 291–299 (2009). https://doi.org/10.1007/s00269-008-0277-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-008-0277-8