Abstract
Background
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has been used as a temporizing procedure to control intra-abdominal or pelvic bleeding. Theoretically, occlusion of the aorta and the resulting ischemia–reperfusion of the lower extremities may increase the risk of extremity compartment syndrome (CS). To date, no study has addressed systematically the incidence and risk factors of CS following REBOA intervention. The purpose of this study was to address this knowledge gap.
Methods
Adult trauma patients from the American College of Surgeons Trauma Quality Improvement Program (ACS-TQIP) database (2016–2019) were included. Patients who received REBOA within 4 h of admission were compared to patients without REBOA after propensity score matching for demographics, vital signs on admission, comorbidities, injury severity of different body regions, pelvic and lower extremity fractures, vascular trauma to the lower extremities, fixation for fractures, angioembolization (AE) for pelvis, preperitoneal pelvic packing (PPP), laparotomy, and venous thromboembolism (VTE) prophylaxis. The primary outcomes were rates of lower extremity CS and fasciotomy and acute kidney injury (AKI). Secondary outcomes included mortality.
Results
There were 534 patients who received REBOA matched with 1043 patients without REBOA. Overall, patients in the REBOA group had significantly higher rates of CS than no REBOA patients [5.4% vs 1.1%, p < 0.001, OR: 5.39]. The risk of CS remained significantly higher in the subgroups of patients with or without pelvic or lower extremity fractures, as well as in the subgroup of patients with associated extremity vascular injury [11.2% vs 1.5%, p < 0.001, OR: 8.12].The fasciotomy and AKI rates were significantly higher in the REBOA group (5.8% vs 1.2%, p < 0.001 and 12.9% vs 7.4%, p< 0.001 respectively).
Conclusion
REBOA use is associated with a higher risk of lower extremity CS, fasciotomy and AKI, especially in patients with associated lower extremity vascular injuries. These complications should be taken into account when considering REBOA use, and close observation for this complication should always be part of the routine monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noncompressible torso hemorrhage is one of the leading causes of death in trauma. [1, 2]. Resuscitative endovascular balloon occlusion of the aorta (REBOA) has been used as a temporizing procedure to control abdominal or pelvic bleeding. Although the concept can be traced back to the Korean war [3], this specific technique reemerged and became popular in the past two decades [4, 5]. Despite the questionable survival benefits, REBOA is currently being used not only for trauma, but also for other causes of abdominal bleeding, such as ruptured aortic aneurysm, post-partum hemorrhage, and gastrointestinal bleeding [6,7,8,9].
Compartment syndrome (CS) of the lower extremities after trauma is a surgical emergency. If it is not treated on time, it can lead to irreversible limb ischemia, with limb loss, AKI or permanent disability. Long bone fracture, crush injury, and prolonged ischemia after vessel injury are well-documented risk factors for CS [10, 11]. To our knowledge, no study has previously addressed systematically the incidence and risk factors of CS following REBOA intervention. We hypothesized that placing REBOA is a risk factor for CS, distinct from the pre-existing trauma. The aim of this study was to evaluate the incidence and risk factors of CS, fasciotomy rates and acute kidney injury (AKI), following placement of REBOA.
Material and methods
This was an ACS-TQIP database study, during the period 2016–2019. All adult (≥ 16-year-old) trauma patients with blunt or penetrating injuries were included. Burns, suffocation, and other mechanisms that could not be classified as blunt or penetrating injury were excluded. Patients who met the following criteria were excluded: cardiac arrest on arrival, died in the emergency department (ED), transferred from other hospitals, non-level 1 or 2 trauma center, head abbreviated injury scale (AIS) > 3, immediate amputation at or above knee level within 4 h. Only patients who survived more than 24 h were included. Patients undergoing ED resuscitative thoracotomy who survived beyond the ED were included.
The included patients were then divided into two cohorts: one received REBOA, and the other did not. REBOA insertion was defined according to the following International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD −10) procedure codes within 4 h: 04L03DZ, 04L03DJ, 04L04DZ, 02LW3DJ, 04L03ZZ, and 04L04ZZ. Fasciotomies of the lower extremities were identified with procedure codes: left upper leg OKNR0ZZ; Right upper leg OKNQ0ZZ; Left lower leg OKNT0ZZ; Right lower leg OKNS0ZZ.
Demographic, epidemiological, and clinical data collected, included age, gender, mechanism, initial vital signs in the ED, body area AIS, injury severity score (ISS), and comorbidities. Other data collected included lower extremity injuries, such as pelvic fracture, femoral fracture, tibial fracture and lower limb vessel injury (iliac, femoral or popliteal vessels). We also recorded specific initial interventions, such as fixation for pelvic, femur or tibial fracture within 24 h, preperitoneal pelvic packing (PPP) within 24 h, laparotomy within 48 h, angioembolization (AE) for pelvis, and pharmacological prophylaxis for VTE. The primary outcome variables were lower extremity CS, fasciotomy after 4 h, amputation at knee or higher levels after 4 h, and AKI. Secondary outcome was survival.
The rate of missing values ranged between 0.0 and 6.4%. The missing pattern was considered missing at random. Multiple imputation was done only for demographic variables missing more than 1.0%, i.e., age, systolic blood pressure, pulse rate, and Glasgow Coma Scale (GCS), by fully conditional specification method with 10 iterations and 5 imputations. Propensity score matching (PSM) was performed for the pooled data after imputation by a 1:2 ratio with nearest matching method. The variables used for matching included age > 55, systolic pressure < 90, pulse rate > 120, GCS < 9, gender, mechanism, and comorbidities showing significant difference before matching. For injury characteristics, we matched head, chest, liver, kidney, spleen, hollow viscus, pelvis, lower limb vessel, femur fracture and tibial fracture with AIS ≥ 3. The injuries of abdomen and lower limb other than those mentioned above were also matched by selecting the most severe injury. To achieve better similarity, interventions including PPP, laparotomy, AE for pelvis, immediate fixation for pelvis/femur/tibia, and VTE prophylaxis were matched. Post-matching balance was defined as standardized mean difference (SMD) less than 0.1 and variance ratio between 0.5 and 2.
Categorial variables were expressed as percentage. Chi-square test or Fisher’s exact test was used for measuring statistical difference. Continuous variables not normally distributed are reported as median with interquartile range while normally distributed ones were reported as mean with standard deviation. Mann–Whitney U test and independent T-test were used accordingly for calculating significance. Statistical significance was set at p < 0.05. All statistics were performed using SPSS 28.0 (IBM Corp., Armonk, NY) and RStudio version 4.2.1 (Boston, MA). The study has been approved by the Institutional Review Board at our institution.
Results
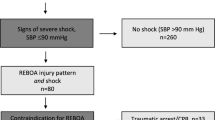
During the four-year study period, there were 1,330,881 adult patients in ACS-TQIP with 798,302 of them meeting our selection criteria. Among all the eligible patients, 594 received REBOA (0.07%) (Fig. 1). In 82% of patients with REBOA, the balloon was placed in zone 3 (between the lowest renal artery and the aortic bifurcation), while 18% were in zone 1 (between the left subclavian artery and the celiac trunk). The demographic, injury characteristics, interventions, and outcomes are listed in Supplement Table 1
After PSM, 534 patients with REBOA were matched to 1043 patients without REBOA (Table 1). The two cohorts were matched in balance since all SMDs were less than 0.1 and the variance ratios were 0.8–1.0. The demographic data, initial vital signs, and comorbidities were similar in both groups. The proportions of AIS > 2 in head, chest, lower extremity, and intraabdominal solid or hollow organs had no significant differences, as well as injury to pelvic, femur, tibial or lower limb vessels. The two cohorts also had similar interventions, including PPP, AE for pelvic fracture, laparotomy, timing of pharmacological VTE prophylaxis, and operative fixation of pelvic or lower extremity long bone fractures.
Overall, patients with REBOA had a significantly higher incidence of CS, and were 5.4 times more likely to develop extremity CS [5.4% vs 1.1% p < 0.001, OR: 5.39 (95% CI 2.67–10.87)] (Table 2). Patients with REBOA placement also had higher rates of amputation [3.6% vs 1.6% p = 0.015, OR: 2.23 (95% CI 1.15–4.32)]. The risk of CS in the REBOA group remained significantly higher in the subgroups of patients with or without fractures of the pelvis, fractures of the femur or the tibia, and with or without associated lower extremity vascular injuries. The risk of CS was particularly high in REBOA patients with lower extremity vascular injuries (11.2% vs 1.5%, p < 0.001, OR: 8.12 (2.26–29.14)]. There was no difference in the incidence of compartment syndrome between zone 1 and zone 3 REBOA placement (5.1% vs 5.5%, p = 0.853) (Table 3). Also, the fasciotomy rates were similar in the two groups (4.0% vs 6.2%, p = 0.405).
Another interesting finding in the study was the significantly higher incidence of AKI in the REBOA group (12.9% vs 7.4%, p < 0.001). Overall, 18 of 40 (45.0%) patients with CS developed AKI, compared to 128 of 1537 (8.3%) patients without CS (p < 0.001). In the subgroup of 29 patients who received REBOA and developed CS, 17 (58.6%) developed AKI, compared to 1 out of 11 patients (9.1%) treated without REBOA that developed CS (p = 0.011).
Finally, the in-hospital mortality was significantly higher in the REBOA patients compared to those who did not receive REBOA (21.9% vs 8.4%, p < 0.001).
Discussion
The role of REBOA in temporary hemorrhage control of noncompressible abdominal or pelvic exsanguination is controversial, with some studies showing improved survival, while others showing no difference or even worse survival [12,13,14,15,16,17]. In the present study, the mortality in the REBOA group was significantly higher than in the control group, which supported the findings of other large database analyses [15,16,17]. However, this finding is in disagreement with other multicenter studies, which have reported improved survival [12,13,14].
The role of REBOA in the presence of severe associated brain trauma is controversial, although recent work suggested that it could be beneficial [18]. However, severe brain trauma may play a major factor in determining outcomes and for this reason we excluded this group of patients.
The purpose and design of this study was specifically to investigate the incidence and risk factors for the development of extremity CS. Our data showed a significantly higher incidence of lower extremity CS in the REBOA group, irrespective of the presence of associated known risk factors. In subgroup analysis, the risk of CS remained higher in REBOA patients with or without pelvic or lower extremity fractures and with or without extremity vascular injuries.
Extremity compartment syndrome after REBOA is rarely mentioned in the literature. Joseph et al. found no significant difference in lower extremity CS between the REBOA and no REBOA groups [17]. Possible explanations for the difference in findings are their inclusion of deaths within 24 h, and that the study did not match specifically for all factors which could be associated with extremity compartment syndrome, such as orthopedic procedures on the pelvis and lower extremities, angioembolization, preperitoneal packing and comorbid conditions. Wasicek et al., in an observational study of 31 REBOA patients and no controls, reported that 3 in 20 patients (15%) with zone 1 and 4 in 11 patients (36%) with zone 3 REBOA developed lower extremity CS [19].
The ischemia produced by the REBOA occlusion of the aorta, followed by the reperfusion injury after deflation of the balloon, produces the highest risk environment for the development of an extremity CS. In the present study, the combination of an extremity vascular injury and a REBOA placement was correspondingly associated with the highest risk of CS (11.2% vs 1.5% in patients without REBOA). The fasciotomy rates were also significantly higher in the REBOA group, especially in patients with associated lower extremity vascular injuries, where 14.7% of patients underwent the procedure, versus only 2.6% in patients without REBOA. The subsequent amputation rate showed a similar trend. REBOA placement was related to higher amputation rate, especially in patients with lower extremity vessel injuries.
The risk of AKI was significantly higher in the REBOA group, confirming similar findings in previous studies [5, 17, 20, 21]. Animal studies have also shown histologic damage to the kidney after placing REBOA in zone 1 [22, 23]. The pathogenesis of AKI after REBOA has largely been attributed to ischemic injury to the kidneys due to the aortic occlusion. However, our findings suggest that rhabdomyolysis due to CS likely also contributes to the development of AKI. Although rhabdomyolysis is not a documented variable in TQIP, we observed that 45% of patients with CS had AKI, while only 8.3% of patients without CS developed AKI. In addition, the combination of REBOA with CS was significantly more deleterious to the kidneys than CS alone. In the subgroup of patients with REBOA and CS, 58.6% developed AKI, compared to only 9.1% of patients with CS but no REBOA (p = 0.011).
A significant strength of this study is the design, which was planned specifically to address the issue of CS after REBOA. The study groups were matched not only for major pelvis and long bone fractures, vascular injury, other body regions injuries, and comorbidities, but also for interventions that may directly or indirectly contribute to lower extremity CS, such as AE for pelvis, fixation for pelvis/femur/tibia, VTE prophylaxis, PPP, and laparotomy.
There are however certain inherent limitations of the study. The TQIP database does not provide specific details related to the REBOA, such as the duration of occlusion or partial/complete occlusion, or the size of the catheter. There is no information about the resuscitation status before and during REBOA placement. The indication and clinical judgement for REBOA vary, individual trauma centers cannot be identified in TQIP, and inter-facility cluster effect and personal surgeon preference cannot be evaluated. We matched the relevant clinical information in the two study groups as well as possible, but only a randomized study would address this limitation. Also, although the database provides information about the zone of the balloon deployment, it is possible that in some cases the balloon was initially inflated in zone 1 and then repositioned to zone 3. We also assumed that the REBOA was properly placed in either zone 1 or 3. However, it is possible that in some patients the REBOA could have been inadvertently placed in the zone 2. The interpretation of outcomes and determination of causality is limited due to the retrospective nature. It is possible, despite the meticulous matching, that the patients receiving REBOA were somehow in more critical condition.
Conclusion
REBOA use in trauma is associated with an increased risk of lower extremity compartment syndrome, fasciotomy, and AKI. These complications should be considered if planning REBOA placement. Further prospective studies are needed to clearly identify the patients who may benefit from REBOA, the optimal duration of balloon deployment, and other strategies to prevent these potential complications.
References
Teixeira G, Inaba K, Hadjizacharia P et al (2007) Preventable or potentially preventable mortality at a mature trauma center. J Trauma 63:1338–1346
Clarke R, Trooskin Z, Doshi J et al (2002) Time to laparotomy for intra-abdominal bleeding from trauma does affect survival for delays up to 90 minutes. J Trauma 52:420–425. https://doi.org/10.1097/00005373-200203000-00002
Hughes W (1954) Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery 36:65–68
Thrailkill A, Gladin H, Thorpe R et al (2021) Resuscitative endovascular balloon occlusion of the aorta (REBOA): update and insights into current practices and future directions for research and implementation. Scand J Trauma Resusc Emerg med 29:8. https://doi.org/10.1186/s13049-020-00807-9
Bukur M, Gorman E, DiMaggio C et al (2021) Temporal changes in REBOA utilization practices are associated with increased survival: an analysis of the AORTA registry. Shock 55:24–32. https://doi.org/10.1097/SHK.0000000000001586
Malina M, Veith F, Ivancev K et al (2005) Balloon occlusion of the aorta during endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther 12:556–559. https://doi.org/10.1583/05-1587.1
Karkos D, Bruce A, Lambert E (2001) Use of the intra-aortic balloon pump to stop gastrointestinal bleeding. Ann Emerg Med 38:328–331. https://doi.org/10.1067/mem.2001.114408
Sano H, Tsurukiri J, Hoshiai A et al (2016) Resuscitative endovascular balloon occlusion of the aorta for uncontrollable nonvariceal upper gastrointestinal bleeding. World J Emerg Surg 11:20. https://doi.org/10.1186/s13017-016-0076-3
Kamijo K, Nakajima M, Shigemi D et al (2022) Resuscitative endovascular balloon occlusion of the aorta for life-threatening postpartum hemorrhage: a nationwide observational study in Japan. J Trauma Acute Care Surg. https://doi.org/10.1097/TA.0000000000003650
Asmar S, Bible L, Chehab M et al (2021) Traumatic femoral artery injuries and predictors of compartment syndrome: a nationwide analysis. J Surg Res 265:159–167. https://doi.org/10.1016/j.jss.2021.03.039
von Keudell G, Weaver J, Appleton T et al (2015) Diagnosis and treatment of acute extremity compartment syndrome. Lancet 386:299–1310. https://doi.org/10.1016/S0140-6736(15)00277-9
Abe T, Uchida M, Nagata I et al (2016) Resuscitative endovascular balloon occlusion of the aorta versus aortic cross clamping among patients with critical trauma: a nationwide cohort study in Japan. Crit Care 20:400. https://doi.org/10.1186/s13054-016-1577-x
Brenner M, Inaba K, Aiolfi A et al (2018) Resuscitative endovascular balloon occlusion of the aorta and resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the american association for the surgery of trauma’s aortic occlusion in resuscitation for trauma and acute care surgery registry. J Am Coll Surg 226:730–740. https://doi.org/10.1016/j.jamcollsurg.2018.01.044
DuBose J, Scalea M, Brenner M et al (2016) The AAST prospective aortic occlusion for resuscitation in trauma and acute care surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg 81:409–419. https://doi.org/10.1097/TA.0000000000001079
Inoue J, Shiraishi A, Yoshiyuki A et al (2016) Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: a propensity score analysis. J Trauma Acute Care Surg 80:559–566. https://doi.org/10.1097/TA.0000000000000968
Norii T, Crandall C, Terasaka Y (2015) Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg 78:721–728. https://doi.org/10.1097/TA.0000000000000578
Joseph B, Zeeshan M, Sakran V et al (2019) Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg 154:500–508. https://doi.org/10.1001/jamasurg.2019.0096
Elkbuli A, Kinslow K, Sen-Crowe B et al (2021) Outcomes of resuscitative endovascular balloon occlusion of the aorta (REBOA) utilization in trauma patients with and without traumatic brain injuries: a national analysis of the american college of surgeons trauma quality improvement program data set. Surgery 70:284–290
Wasicek J, Teeter A, Yang S et al (2018) Life over limb: lower extremity ischemia in the setting of resuscitative endovascular balloon occlusion of the aorta (REBOA). Am Surg 84:971–977
Mikdad S, van Erp M, Moheb E et al (2020) Pre-peritoneal pelvic packing for early hemorrhage control reduces mortality compared to resuscitative endovascular balloon occlusion of the aorta in severe blunt pelvic trauma patients: a nationwide analysis. Injury 51:1834–1839. https://doi.org/10.1016/j.injury.2020.06.003
Bini K, Hardman C, Morrison J et al (2022) Survival benefit for pelvic trauma patients undergoing resuscitative endovascular balloon occlusion of the aorta: results of the AAST aortic occlusion for resuscitation in trauma acute care surgery (AORTA) registry. Injury 53:2126–2132
Kauvar S, Schechtman W, Thomas B et al (2019) Effect of partial and complete aortic balloon occlusion on survival and shock in a swine model of uncontrolled splenic hemorrhage with delayed resuscitation. J Trauma Acute Care Surg 87:1026–1034. https://doi.org/10.1097/TA.0000000000002439
Russo M, White M, Baer G (2021) Partial resuscitative endovascular balloon occlusion of the aorta: a systematic review of the preclinical and clinical literature. J Surg Res 262:101–114. https://doi.org/10.1016/j.jss.2020.12.054
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have nothing to declare.
Informed consent
Informed consent is exempted.
Human and animal rights
The study has been approved by the Institutional Review Board at University of Southern California Institutional Review Board (HS-22-00465).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, YT., Lewis, M.R., Arase, M. et al. Resuscitative Endovascular Balloon Occlusion of the Aorta is Associated with Increased Risk of Extremity Compartment Syndrome. World J Surg 47, 796–802 (2023). https://doi.org/10.1007/s00268-022-06832-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06832-2