Abstract
Background
Immunotherapy advances for the treatment of cutaneous melanoma question its efficacy in treating anorectal mucosal melanoma (ARMM). We aimed to identify the prevalence, current management, and overall survival (OS) for ARMM.
Methods
Review of patients with ARMM from 2004 to 2015 National Cancer Database. Factors associated with immunotherapy were identified using multivariable logistic regression. The primary outcome was 2- and 5-year OS. Subgroup analysis by treatment type was performed.
Results
A total of 1331 patients were identified with a significant increase in prevalence (2004: 6.99%, 2015: 10.53%). ARMM patients were older, white, on Medicare, and from the South. The most common treatment was surgery (48.77%), followed by surgery + radiation (11.75%), surgery + immunotherapy (8.68%), and surgery + chemotherapy (8.68%). 16.93% of patients received immunotherapy, with utilization increasing (7.24%: 2004, 21.27%: 2015, p < 0.001). Patients who received immunotherapy had a significantly better 2-year OS (42.47% vs. 49.21%, p < 0.001), and other therapies did not reveal a significant difference. Adjusted analysis showed no difference in 2- and 5-year OS based on therapy type.
Conclusion
The prevalence of ARMM has increased. The use of immunotherapy has increased substantially. Some survival benefit with the administration of immunotherapy may exist that has yet to be revealed. A more aggressive treatment paradigm is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anorectal mucosal melanoma (ARMM) is a rare, poorly understood, and highly lethal malignancy. Five-year overall survival (OS) is dismal, ranging between 20 and 22%, with disease-free survival of 16% to 17% [1,2,3,4,5,6,7,8]. ARMM accounts for between 1 and 2% of lower gastrointestinal malignant lesions, 1.3% of all melanomas, and 16.5% of mucosal melanomas [1, 2, 9]. Mucosal melanomas (MM) arise from non-hair-bearing mucosal surfaces of the body and themselves account for only 1–2% of all melanomas in the USA, the majority being cutaneous melanoma (CM) [10]. MM have been shown to be both clinically and genetically distinct from CM [11, 12], with far worse stage-matched prognosis [13].
The rarity of ARMM has restricted prior studies to relatively small patient cohorts, with the largest study to date containing a sample size of only 260 patients [14]. As with any rare disease, it is subsequently difficult to determine the optimal management and therapeutic protocols. Much debate has previously been held as to the extent of surgical debridement, whether or not a full lymph node dissection should be performed, and the utility of neoadjuvant and adjuvant therapies. Current data suggest that there is no difference in OS after local resection or radical resection [2, 15, 16], and that lymphadenectomy also has no positive impact on survival [17]. Although the effect of traditional adjuvant radiation therapy has been questioned and suggested not to improve survival [18], there have been recent endeavors to develop and improve additional systemic therapies, including chemotherapy, immunotherapy, target therapy, and antiangiogenic therapies. Some success has been observed in patients with CM, with the FDA first approving IL-2 blockers for use in malignant melanoma in 199 [8, 19]; however, this has yet to be seen in ARMM or in other forms of MM.
No study to date has looked at a large sample of ARMM patients and compared their OS based upon their definitive management. This study aims to investigate the prevalence of ARMM and to assess the trends in practice.
Materials and methods
Data source
This was a retrospective analysis using the National Cancer Database (NCDB) from 2004 to 2015. This nationwide clinical oncology database is sponsored by the American College of Surgeons and the American Cancer Society, and captures over 70% of newly diagnosed cancer patients from more than 1500 Commission on Cancer (CoC) accredited facilities [20]. The Johns Hopkins University School of Medicine Institutional Review Board approved this study.
Inclusion and exclusion criteria
Adults diagnosed with ARMM (International Classification of Diseases for Oncology, 3rd edition [ICD-0 3] histology codes of 8720, 8721, 8722, 8723, 8730, 8743, 8745, 8746, 8770, 8771, and 8772) were included in the study. Patients with missing information on treatment type were excluded. Patients were stratified based on treatment type.
Baseline characteristics
Patient demographic characteristics included age at diagnosis (<50, 50–59, 60–69, 70–79, ≥80), sex, race (White, Black, Other, or Unknown), Spanish/Hispanic ethnicity, education, median household income, medical insurance status (private, Medicare, Medicaid, other government, none, or unknown), population density of patient residence (metro, urban, rural, or unknown), and distance from patient’s zip code to location of admitting hospital (<5, 5–10, 10–25, or ≥25 miles). Patient education and income were used as proxy for patient’s socioeconomic status and were estimated by matching patient’s zip code of residence with the American Community Survey data. The NCDB reports education as a percentage of no high school completion with the following predetermined categories: <7%, 7–12.9%, 13–20.9%, or ≥21%. Income is adjusted for 2012 inflation and categorized as less than $38,000, $38,000-$47,999, $48,000–62,999, or more than $63,000.
Patient clinical and oncologic characteristics included Charlson–Deyo Score used as surrogate for patients comorbidities (0, 1, or ≥2), nodes (negative, positive, or unknown), tumor size (<3, 3–4.9, or ≥5 cm), and margins (negative, positive, or unknown). Treatment types included immunotherapy, surgery, chemotherapy, and radiation therapy—all treatment types were administered in no particular sequence and at any point in time following the diagnosis. Treatment comparisons were performed for: immunotherapy versus no immunotherapy, surgery alone versus surgery + immunotherapy, surgery alone versus surgery + other treatment, and for no surgery versus local excision versus radical resection. Information related to cancer stage and grade differentiation was omitted due to missing data.
Hospital characteristics included hospital type (community cancer program, academic/research program including NCI-designated comprehensive cancer centers, integrated network cancer program, or unknown) and hospital geographic region that was defined according to the Census regions and division of the USA (Northeast, South, Midwest, West, or unknown) [21].
Outcomes and statistical analysis
The primary outcome was 2- and 5-year OS. Pearson’s χ2 test was used for categorical variables. Multivariable logistic regression analysis was used to identify factors associated with administration of immunotherapy and included variables with p < 0.25 in univariate analysis as recommended by Hosmer and Lemeshow [22]. OS was defined as “time in months from diagnosis to either death or last follow-up date.” Note that, in the NCDB, vital status is only available for patients diagnosed up to and including year 2014. Survival analysis was performed for all patients and for each treatment type. Kaplan–Meier method with log-rank test was utilized to compare survival curves between treatment groups. Cox proportional hazard model was used to examine the impact of treatment type on OS while adjusting for other factors. Statistical significance was considered as p < 0.05. Statistical analysis was performed with Stata/MP version 14 (StataCorp LP, College Station, TX, USA).
Results
Study cohort
Between 2004 and 2015, the NCDB captured 412,662 anorectal (anal, anal canal, anorectum, rectum, and rectosigmoid junction) cancer diagnoses in the USA, including 1333 melanoma cases analyzed in this study. The diagnosed patients were predominately older (median of 68 years), white (87.97%), on Medicare (51.26%), and from the South (36.55%).
Prevalence and treatment trends
A significant increase in prevalence of ARMM has been observed (from 6.99% in 2004 to 10.53% in 2015, p < 0.001; denominator being total number of ARMM diagnoses in the NCDB between 2004 and 2015). Out of all treatment types, the most common treatment was surgery alone (48.77%), followed by surgery with radiation (11.75%), surgery with immunotherapy (8.68%), and surgery with chemotherapy (8.68%) (Fig. 1). Utilization of chemotherapy and radiation therapy has been consistent throughout the last decade; however, surgery and, especially, immunotherapy have been on the rise (7.18% in 2004 to 9.76% in 2015, p < 0.01; 7.24% to 21.27%, p < 0.001; respectively) (Fig. 2). While surgical treatment increased gradually, immunotherapy increased rapidly starting in 2013.
Factors associated with immunotherapy
A total of 221 (16.93%) patients received immunotherapy, with the median time from diagnosis to administration of immunotherapy of 76 days (IQR 53–119 days). Immunotherapy was recommended, but not administered to 15 patients due to either their or their family member/guardian refusal. Immunotherapy was contraindicated in 14 patients due to patient risk factors (i.e., comorbid conditions, advanced age). Patients who received immunotherapy tended to be younger, have private insurance, and be treated in an academic/research hospital (Table 1). After adjusting for other factors, younger age (≥80-ref; >50 OR 8.41, 95% CI 3.87–18.26, p < 0.001; 50–59: OR 3.86, 95% CI 1.89–7.88, p < 0.001; 60–69: OR 4.21, 95% CI 2.12–7.99, p < 0.001; and 70–79: OR 1.93, 95% 1.01–3.70, p = 0.047), positive nodes (negative-ref; OR 1.99, 95% CI 1.19–3.34, p < 0.01), and larger tumor size (<3 cm-ref; 3–4.9 cm: OR 1.63, 95% CI 1.05–2.55, p = 0.03; ≥5 cm: OR 1.66, 95% CI 1.04–2.65, p = 0.03) were identified as independent predictors of immunotherapy treatment (Table 2).
Overall survival
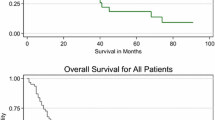
Two- and five-year OS rates were 43.57% and 20.50%, respectively, with median OS of 19.35 months. Patients who received immunotherapy had significantly better 2-year OS rates (49.21% vs. 42.47%, respectively, p = 0.03) than those without immunotherapy (Fig. 3). However, 5-year OS was comparable between patients with and without immunotherapy (19.18% vs. 20.73%, p = 0.23). Patients who received immunotherapy lived about 5 months longer than those who did not (23.36 months vs. 18.56 months). Comparison of surgery alone versus surgery and immunotherapy, and surgery alone versus surgery and other treatments did not show any significant advantage of one treatment over the other in 2- and 5-year OS (Figs. 4, 5). Having any surgical intervention (local excision or radical resection) offered better OS than having none (Fig. 6). However, there was no significant difference in either 2- or 5-year OS between the local excision and radical resection (p = 0.28 and p = 0.89, respectively). Consistent with the Kaplan–Meier results, unadjusted Cox proportional hazard analysis revealed that patients who received immunotherapy had significantly better 2-year OS (HR 0.78, 95% CI 0.62–0.97, p = 0.03), and patients undergoing surgical intervention had better 2 and 5-year OS when compared with non-surgical patients (Table 3). The latter was seen even after adjusting for other factors; however, adjusted analysis showed no difference in 2- and 5-year OS between the other studied treatment types.
Discussion
To our knowledge, this is the largest retrospective cohort analysis of patients with ARMM, with 1333 patients analyzed. A significant increase in the prevalence of ARMM is observed between 2004 and 2015, a finding which is mirrored in previous epidemiological studies [14]. Although it is unclear as to whether this increase in ARMM prevalence represents improved screening and detection, this trend has interestingly been shown to mirror the increase in CM diagnoses [23, 24]. Although the age, race, and gender observations reflect prior studies, the correlation with latitude is something that has been strongly debated. Callahan and colleagues discovered a positive trend of ARMM with Southern latitude in their 2015 study [14]; however, Weinstock found a significant association of ARMM with Northern latitude [23]. Meanwhile, a Norwegian study did not demonstrate any association between latitude and ARMM [25]. As such, the jury remains out as to whether latitude plays a real impact in the development of ARMM.
This study is the first, to the authors’ knowledge, to examine the management practice for patients diagnosed with ARMM and to scrutinize the factors associated with management selection. Surgery remains the most common treatment, either alone or in combination with radiation therapy, chemotherapy, or immunotherapy. The data on the subtype of surgery support the findings from multiple prior studies demonstrating no survival benefit with any one particular surgical approach [2, 15, 26]. What is of interest is the significant increase in immunotherapy treatment, with 7.24% of patients receiving immunotherapy in 2004, compared to 21.27% of patients in 2015. These trends likely reflect the development and success of immunological checkpoint blockade and target therapies for management of malignant CM. Ipilimumab, an antibody to cytotoxic T-lymphocyte-associated protein-4, was the first agent shown to improve OS in a phase-III, randomized control trial of patients with malignant melanoma [27, 28]. In addition to ipilimumab, inhibitors of mutant B-RAF, MEK-1/2, and KIT have also been developed successfully for the management of malignant melanoma. Dabrafenib, a reversible ATP-competitive inhibitor that selectively inhibits B-RAF, has demonstrated significantly increased OS in patients with mutant B-RAF [29]. C-Kit expression has been shown by immunohistochemistry to exist in most melanomas. Although early studies of c-Kit blockers such as imatinib were unsuccessful in the treatment of malignant melanoma [30, 31], imatinib, sunitinib, sorafenib, dasatinib, and other c-Kit blockers have demonstrated activity against malignant MM, which is thought to be due to an increased frequency of KIT aberrations of amplifications in MM compared to CM [11, 32,33,34,35,36,37]. Further success has been seen with the use of anti-PD1, anti-PDL 1, and anti-PDL 2 drugs in metastatic melanoma. Le Min reported one case of anti-PD1 treatment in a patient with advanced MM with a durable near-complete response [38], and a further case report by Tokuhara and colleagues also showed a marked response to PD1 inhibitors [39]. Despite the success experienced in these case reports and small studies, some anecdotal evidence of rapid disease progression with the use of immunological therapies has been reported in the literature [40]. This further highlights the value of this article and the need for long-term observation of the effect of immunotherapy upon survival in MM.
Our results suggest that those patients most likely to receive immunotherapy as part of their treatment regimen include those who are younger, with larger tumors, and positive lymph nodes. This suggests that immunotherapy is being utilized and/or reserved for those patients with more advanced disease. Overall, 2-year and 5-year OS rates were 43.57% and 20.50%, respectively, with a median OS of 19.35 months. Those patients who did receive immunotherapy had significantly better 2-year OS compared to those who did not receive immunotherapy (49.21% vs. 42.47%, Fig. 3), and on average lived about 5 months longer (23.36 vs. 18.56 months). Despite this, 5-year OS was comparable between patients treated with and without immunotherapy, suggesting that the benefits of current immunotherapy regimens and drugs may be observed only in the short term. What is important to note is that the data from the NCDB regarding vital status are only available for patients diagnosed up to and including 2014, which means the effects of the large increase in immunotherapy use in 2015 are not reflected at present. When comparing surgery alone with surgery + immunotherapy or surgery + additional therapy, no significant advantage was observed in 2- or 5-year OS (Figs. 4, 5).
The current strategy of surgery with or without neoadjuvant or adjuvant therapy results in a dismal OS, with only 20% surviving to 5 years [41]. The aggressive nature of ARMM and the continued poor outcomes, with no improvement observed over the past five decades [42], warrants a new approach to management. Efforts at screening for CM have resulted in diagnosis at earlier stages and improved survival; however, no such advances have been made in MM due to the rarity of the disease. What is clear is that ARMM is a heterogeneous disease, with many potential molecular targets for chemotherapy. The observed improvement in 2-year OS with immunotherapy seen in this study may suggest that the benefits of this approach are just beginning to surface, and that with more time, a greater effect may be apparent. An individualized approach to treatment is warranted, with molecular analysis to identify the appropriate target therapy.
This study has several limitations that are important to discuss. Firstly, this is a retrospective review of a data that is drawn from the NCDB database that relies on accurate coding of Current Procedural Terminology codes. Unfortunately, the tumor grade or stage of the ARMM was not recorded in the database, which limits the ability to see whether treatment strategy varies by stage. The finding of tumor size being a predictor for immunotherapy protocol should be interpreted with caution, as 30% of patients had no information on the size of the tumor. In addition, as previously mentioned, there are no data on vital status for 2015, which restricts analysis of the longer-term effect of immunotherapy. A further limitation lies in the lack of detail on the subtype, or dosage, of any immunotherapy or pharmaceutical agents used to treat MM. Despite all these limitations, the numerous strengths of the NCDB overcome many of the weaknesses of administrative or billing datasets. First of all, NCDB is the largest colorectal cancer database in the world [43]. Secondly, it contains more detailed information on specific clinical cancer pathology, treatment, hospital level factors, and sociodemographic factors.
Conclusion
This study confirms that the 2- and 5-year OS for ARMM remains dismal, despite the development and increase in the use of adjuvant therapies. While surgery remains the optimal treatment option, it is in essence a palliative procedure, with no significant improvement in OS observed with the addition of any adjuvant therapy. The utilization of immunotherapy has increased in the past few years, and 2-year OS is significantly enhanced compared to those who do not receive the treatment. Immunotherapy does not appear to impact 5-year OS; however, more time is required to see whether the survival is impacted, hopefully shifting the management of ARMM from palliative to curative.
A change in paradigm in the management of ARMM is necessary in an attempt to improve survival. Personalized molecular analysis should be performed to identify patients with specific genetic mutations that might be an appropriate target for immunotherapeutic agents or specific target therapies, which have shown promise in early clinical trials.
References
Chang AE, Karnell LH, Menck HR (1998) The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83:1664–1678
Bullard KM, Tuttle TM, Rothenberger DA, Madoff RD, Baxter NN, Finne CO, Spencer MP (2003) Surgical therapy for anorectal melanoma. J Am Coll Surg 196:206–211
Meguerditchian AN, Meterissian SH, Dunn KB (2011) Anorectal melanoma: diagnosis and treatment. Dis Colon Rectum 54:638–644
Moozar KL, Wong CS, Couture J (2003) Anorectal malignant melanoma: treatment with surgery or radiation therapy, or both. Can J Surg 46:345–349
Row D, Weiser MR (2009) Anorectal melanoma. Clin Colon Rectal Surg 22:120–126
Brady MS, Kavolius JP, Quan SH (1995) Anorectal melanoma. A 64-year experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum 38:146–151
Yeh JJ, Shia J, Hwu WJ, Busam KJ, Paty PB, Guillem JG, Coit DG, Wong WD, Weiser MR (2006) The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann Surg 244:1012–1017
Yeh JJ, Weiser MR, Shia J, Hwu WJ (2005) Response of stage IV anal mucosal melanoma to chemotherapy. Lancet Oncol 6:438–439
Hillenbrand A, Barth TF, Henne-Bruns D, Formentini A (2008) Anorectal amelanotic melanoma. Colorectal Dis 10:612–615
McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW (2005) Incidence of noncutaneous melanomas in the US. Cancer 103:1000–1007
Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, Pavlick AC, Fusco A, Cane L, Takebe N, Vemula S, Bouvier N, Bastian BC, Schwartz GK (2011) KIT as a therapeutic target in metastatic melanoma. JAMA 305:2327–2334
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC (2005) Distinct sets of genetic alterations in melanoma. N Engl J Med 353:2135–2147
Carvajal RD, Spencer SA, Lydiatt W (2012) Mucosal melanoma: a clinically and biologically unique disease entity. J Natl Compr Cancer Netw 10:345–356
Callahan A, Anderson WF, Patel S, Barnholtz-Sloan JS, Bordeaux JS, Tucker MA, Gerstenblith MR (2016) Epidemiology of anorectal melanoma in the United States: 1992 to 2011. Dermatol Surg 42:94–99
Kiran RP, Rottoli M, Pokala N, Fazio VW (2010) Long-term outcomes after local excision and radical surgery for anal melanoma: data from a population database. Dis Colon Rectum 53:402–408
Pessaux P, Pocard M, Elias D, Duvillard P, Avril MF, Zimmerman P, Lasser P (2004) Surgical management of primary anorectal melanoma. Br J Surg 91:1183–1187
Ciarrocchi A, Pietroletti R, Carlei F, Amicucci G (2017) Extensive surgery and lymphadenectomy do not improve survival in primary melanoma of the anorectum: results from analysis of a large database (SEER). Colorectal Dis 19:158–164
Tchelebi L, Guirguis A, Ashamalla H (2016) Rectal melanoma: epidemiology, prognosis, and role of adjuvant radiation therapy. J Cancer Res Clin Oncol 142:2569–2575
Sadozai H, Gruber T, Hunger RE, Schenk M (2017) Recent successes and future directions in immunotherapy of cutaneous melanoma. Front Immunol 8:1617
(2017) National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb
(2017) Census regions and divisions of the United states. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
Hosmer DW Jr, Lemeshow S (2000) Applied logistic regression, 2nd edn. Wiley, New York
Weinstock MA (1993) Epidemiology and prognosis of anorectal melanoma. Gastroenterology 104:174–178
Cote TR, Sobin LH (2009) Primary melanomas of the esophagus and anorectum: epidemiologic comparison with melanoma of the skin. Melanoma Res 19:58–60
Micu E, Juzeniene A, Moan J (2011) Comparison of the time and latitude trends of melanoma incidence in anorectal region and perianal skin with those of cutaneous malignant melanoma in Norway. J Eur Acad Dermatol Venereol 25:1444–1449
Iddings DM, Fleisig AJ, Chen SL, Faries MB, Morton DL (2010) Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patients? Ann Surg Oncol 17:40–44
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD (2010) Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 116:1767–1775
Gorka E, Fabo D, Gezsi A, Czirbesz K, Fedorcsak I, Liszkay G (2018) Dabrafenib therapy in 30 patients with melanoma metastatic to the brain: a single-centre controlled retrospective study in Hungary. Pathol Oncol Res 24:401–406
Kim KB, Eton O, Davis DW, Frazier ML, McConkey DJ, Diwan AH, Papadopoulos NE, Bedikian AY, Camacho LH, Ross MI, Cormier JN, Gershenwald JE, Lee JE, Mansfield PF, Billings LA, Ng CS, Charnsangavej C, Bar-Eli M, Johnson MM, Murgo AJ, Prieto VG (2008) Phase II trial of imatinib mesylate in patients with metastatic melanoma. Br J Cancer 99:734–740
Ugurel S, Hildenbrand R, Zimpfer A, La Rosee P, Paschka P, Sucker A, Keikavoussi P, Becker JC, Rittgen W, Hochhaus A, Schadendorf D (2005) Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer 92:1398–1405
Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, Jagannathan J, Van den Abbeele AD, Velazquez EF, Demetri GD, Fisher DE (2008) Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol 26:2046–2051
Lutzky J, Bauer J, Bastian BC (2008) Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res 21:492–493
Kluger HM, Dudek AZ, McCann C, Ritacco J, Southard N, Jilaveanu LB, Molinaro A, Sznol M (2011) A phase 2 trial of dasatinib in advanced melanoma. Cancer 117:2202–2208
Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, Fermeglia M, Gopal YN, Yang D, Podoloff DA, Ivan D, Kim KB, Papadopoulos N, Hwu P, Mills GB, Davies MA (2009) Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther 8:2079–2085
Zhao J, Zhu Y, Zhang C, Wang X, He H, Wang H, Wu Y, Zhou W, Shen Z (2013) Sorafenib or sunitinib as postoperative adjuvant therapy for Chinese patients with locally advanced clear cell renal cell carcinoma at high risk for disease recurrence. Urol Oncol 31:1800–1805
Quintas-Cardama A, Lazar AJ, Woodman SE, Kim K, Ross M, Hwu P (2008) Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat Clin Pract Oncol 5:737–740
Min L, Hodi FS (2014) Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res 2:15–18
Tokuhara K, Nakatani K, Tanimura H, Yoshioka K, Kiyohara T, Kon M (2017) A first reported case of metastatic anorectal amelanotic melanoma with a marked response to anti-PD-1 antibody nivolumab: a case report. Int J Surg Case Rep 31:188–192
Faure M, Rochigneux P, Olive D, Taix S, Brenot-Rossi I, Gilabert M (2018) Hyperprogressive disease in anorectal melanoma treated by PD-1 inhibitors. Front Immunol 9:797
Malaguarnera G, Madeddu R, Catania VE, Bertino G, Morelli L, Perrotta RE, Drago F, Malaguarnera M, Latteri S (2018) Anorectal mucosal melanoma. Oncotarget 9:8785–8800
Kirchoff DD, Deutsch GB, Foshag LJ, Lee JH, Sim MS, Faries MB (2016) Evolving therapeutic strategies in mucosal melanoma have not improved survival over five decades. Am Surg 82:1–5
MacCallum C, Skandarajah A, Gibbs P, Hayes I (2018) The value of clinical colorectal cancer registries in colorectal cancer research: a systematic review. JAMA Surg 153:841–849
Funding
Mr. Edwin Lewis provided generous support to Dr. Efron’s Department of Surgery Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of this paper have any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimers: The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Rights and permissions
About this article
Cite this article
Taylor, J.P., Stem, M., Yu, D. et al. Treatment Strategies and Survival Trends for Anorectal Melanoma: Is it Time for a Change?. World J Surg 43, 1809–1819 (2019). https://doi.org/10.1007/s00268-019-04960-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-04960-w