Abstract
Background
Laparoscopic partial splenectomy (LPS) is a challenging procedure. The aim of this review was to evaluate its feasibility, safety, and potential benefits.
Methods
We conducted a comprehensive review for the years 1995–2018 to retrieve all relevant articles.
Results
A total of 44 studies with 252 patients undergoing LPS were reviewed. Six studies described combined operations. Ranges of operative time and estimated blood loss were 50–225 min and 0–1200 ml, respectively. There are eight patients need blood transfusion in 231 patients with available data. The conversion rate was 3.6% (9/252). Overall, 27 patients (10.7%;27/252) developed postoperative or intraoperative complications. Overall mortality was 0% (0/252). The length of postoperative stay (POS) varied (1–11 days). Among four comparative studies, one showed LPS could reduce POS than laparoscopic total splenectomy (LTS) (LTS 5.4 ± 1.8 days, LPS 4.2 ± 0.8 days, p = 0.027) and complications (pleural effusion (LTS 9/22, LPS 0/15, p = 0.005), splenic vein thrombosis (LTS 10/22, LPS 0/15, p = 0.002)). Another comparative study showed LPS may benefit emergency patients. However, one comparative study showed LPS was associated with more pain, longer time to oral intake, and longer POS in children with hereditary spherocytosis. The fourth comparative study showed robotic subtotal splenectomy was comparable to laparoscopy in terms of POS and complication. The main benefits were lower blood loss, vascular dissection time, and a better evaluation of splenic remnant volume.
Conclusions
In early series of highly selected patients, LPS appears to be feasible and safe when performed by experienced laparoscopic surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For decades, the unnecessary roles of spleen have led surgeons to remove the total spleen without hesitation until a retrospective analysis of 2796 splenectomy cases managed in the 1970s showed that septic infections developed in 119 patients (4.25%) and that 71 of these patients (60%) succumbed to their infections [1]. With the better understanding of the importance of the spleen as an important organ of immune system and of the long-term complications in terms of total splenectomy [2,3,4], more and more surgeons prefer to parenchyma-preserving surgical procedures. Partial splenectomy is a good method to prevent post-splenectomy infections by preservation of the immunologic role of the spleen [5, 6]. The first successful partial splenectomy through open approach was reported in 1980 by Morgenstern and Shapiro [7]. However, LPS is still a challenging procedure. One major difficulty when considering LPS is the risk of intraoperative and/or postoperative bleeding. Nevertheless, with the development of technology and understanding of the end-vascular distribution of intrasplenic vessels, LPS was possible. We knew later that the splenic artery branches in superior and inferior polar arteries, which further divide into several segmental intrasplenic end arteries [8, 9]. The first LPS procedure was performed in 1995 by Uranus et al. in pigs [10]. Poulin et al. [11] reported the first case of LPS for ruptured spleen in 1995. Today, LPS is being increasingly advocated and recommended, and even the first case of single-port LPS has been reported by Tae Ho Hong in 2010 [12]. The aim of this review was to evaluate the feasibility, safety, and potential benefits of LPS.
Materials and methods
Literature search strategies
A systematic search of the scientific literature was carried out using the PubMed, relevant online journals, and the Internet for the years 1995–2018 to obtain access to all publications involving LPS for humans. Searches were conducted restricted to English in language. To avoid duplication of data, articles from the same unit or hospital were included only once if data were being updated in a later publication. The search terms were “laparoscopic partial splenectomy,” “laparoscopic subtotal splenectomy,” “laparoscopic splenic surgery,” “Robotic partial splenectomy,” “Robot-assisted partial splenectomy,” “Robotic subtotal splenectomy.” The search strategy applied to PubMed is listed as below: ((((laparoscopic partial splenectomy) OR laparoscopic subtotal splenectomy) OR Robotic partial splenectomy) OR Robot-assisted partial splenectomy) OR Robotic subtotal splenectomy. All available major publications from the past 24 years were considered.
Inclusion criteria
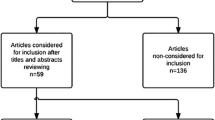
Articles were selected if the abstract contained data of patients who underwent LPS for splenic diseases in the form of case reports, controlled or comparative studies, and articles about summary of experience. Conference abstracts were included if they contained relevant data. The reference lists of these articles were also reviewed to find additional candidate studies. In the case of duplicate publications, the latest and most complete study was included. Letter articles or review articles were excluded from this study. Data extracted for this study were taken from the published reports; authors were not contacted to obtain additional information. All articles selected for review of full text were distributed to two reviewers (Y.F. and G.L), who independently decided on inclusion/exclusion and independently abstracted the study data. Any discrepancies in agreement were resolved by consensus. The flowchart of this selection process is summarized in Fig. 1. IRB approval was not needed for this paper.
Results
Using the search strategy mentioned above, a total of 60 potentially relevant citations were found. We excluded two irrelevant articles (one letter article and one only surgical technique description) and two non-English articles by review of titles and abstracts. Forty-nine publications were selected for review of full text and three duplicate publications, one article undergone LPS for pigs not human, and eight articles that we could not get detailed data were excluded from our review. Forty-four [5, 6, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] with a total of 252 patients undergoing LPS or LSS met the criteria for analysis. These included four case-matched comparative studies. There were no RCTs or meta-analyses.
Indications and procedures of LPS
Indications for LPS varied in these series (Table 1). The most common indications in these series were splenic cystic lesions(n = 84) [5, 6, 12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30, 51, 52], followed by splenic hematological diseases (n = 70) [5, 6, 21, 24, 31, 33,34,35], non-cystic intraparenchymal lesions (n = 59) [5, 6, 20,21,22,23,24, 36,37,38,39,40,41,42,43,44, 50, 52], spleen rupture (n = 22) [11, 45], splenomegaly of unknown origin (n = 9) [5], splenic abscess (n = 3) [23, 42, 49], severe splenic pain due to ischemia provoked by vascular obstruction of the spleen (n = 2) [47], and each for Gandy–Gamna bodies, Benign metaplasia, and undiagnosed splenic lesion [22, 48]. The most common surgical procedures performed in these series were four-trocar laparoscopic splenectomy (n = 117) [14, 16,17,18, 20, 22,23,24, 28, 30, 31, 35, 38, 39, 42, 43, 45, 47, 48, 50, 51], followed by three-trocar laparoscopic splenectomy (n = 53) [5, 15, 19, 21, 26, 27, 33], five-trocar laparoscopic splenectomy (n = 47) [11, 13, 31, 34, 41, 46, 49, 52], two-trocar laparoscopic splenectomy (n = 4) [39, 40], single-incision laparoscopic splenectomy(n = 2) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29], and hand-assisted laparoscopic partial splenectomy (n = 1) [36]. Six studies described combined operations including 16 cases of cholecystectomy [6, 31, 32, 34], case of esophagogastric devascularization [35], and one case of hepatic hydatid cyst excision [29].
Operative parameters (operative time, blood loss, blood transfusion, conversion, etc.)
Various operative parameters are summarized in Table 2. The range of operative times of LPS (including combination operation) procedure was 50–225 min (n = 37 studies) [5, 6, 11,12,13,14, 16,17,18, 20,21,22,23,24,25,26, 28, 29, 31,32,33,34,35,36, 38,39,40,41,42,43,44,45,46, 48,49,50,51,52]. The range of estimated blood loss (EBL) was 0–1200 ml (n = 39 studies) [6, 11,12,13,14,15,16,17,18, 20,21,22,23,24,25,26, 28,29,30,31,32,33,34,35,36, 38,39,40,41,42,43,44,45,46, 48,49,50,51,52]. There are eight patients need blood transfusion in 231 patients with available data (except Li et al. [45] with no available data which contained 21 patients diagnosed with splenic rupture). Among all 252 cases eligible in the current review, a total of five cases (1.98%) were converted to laparoscopic total splenectomy [20, 22, 33, 45], but one was happened 2 years later after LPS [33]. Three cases (1.19%) were converted to open partial splenectomy [5, 22], and 1 case (0.40%) was converted to open total splenectomy 11 months later after LPS [34]. Main reasons of conversion to LTS in these cases were as follows: subsequent unstable vital sign during LPS in two cases [45], hemorrhage in the splenic artery as a result of failure to fire the stapler in one case [20], fresh-frozen tissue examination could not overrule malignancy in one case [22] ,one case developed splenic regrowth accompanied by worsening hemolysis and anemia 2 years later [33]. The reasons of conversion to open partial splenectomy were bleeding [5] or pneumothorax [22] resulting from dissection of inflammatory adhesions between the spleen and the diaphragm. One patient required open splenic remnant removal 11 months after initial surgery due to persistent mild hemolytic anemia and adhesion of the splenic remnant [34]. There was one intraoperative complication (a small bowel tear) during spleen extraction, and then, the portion of small bowel was resected with a functional end-to-end stapled anastomosis.
Resected specimen
For 60 children with hereditary spherocytosis [6, 31,32,33,34], they underwent laparoscopic subtotal splenectomy. The remnant spleen size was 10–30% [32,33,34],with upper pole preserved in 40 patients [6, 31,32,33,34] and lower pole preserved in 20 patients [6, 34].
Perioperative mortality
None perioperative death was observed among all studies.
Morbidity, reoperation and hospital stay
Postoperative morbidities varied across studies (0–33.3%). Overall, 27 patients (10.71%;27/252) developed complications. Postoperative fluid collection occurred in 15 cases [5, 6, 20, 22, 28, 34, 35, 45]. Among them, one patient suffered from intraperitoneal fluid collection requiring radiological drainage [22], one patient got left subphrenic fluid collection which could not be approached percutaneously and required a laparoscopic drainage [28], and others were treated conservatively. Postoperative wound infection occurred in two cases [33, 34] without special treatment. Postoperative portal vein thrombosis occurred in one case [20] and underwent laparoscopic total splenectomy. Postoperative pulmonary embolism occurred in one case [22] and required prolonged anticoagulation. Postoperative ileus occurred in one case [33] who was treated with nasogastric tube decompression and resolved after 3 days. Postoperative atelectasis occurred in two cases [23] without further treatment. Postoperative thrombocytosis occurred in one case [23] and required taking aspirin orally. Intraoperative small bowel tearing during spleen extraction occurred in one case [33], and the portion of small bowel was resected, and then, a functional end-to-end stapled anastomosis was fashioned. No bleeding complication occurred. In 35 studies with available data [5, 6, 11, 12, 14,15,16,17,18,19,20,21, 23,24,25,26, 28,29,30,31, 33,34,35,36, 38,39,40, 43, 44, 46,47,48,49, 51, 52], the average/median length of POS also varied from 1 to 11 days across reports. Notably, 14 studies [11, 14, 16, 17, 19, 25, 26, 30, 38, 40, 44, 47, 48, 51] reported less than or equal to 3 days of average/median POS in their series.
Comparison of short-term outcomes between LPS and LTS
There were only three reports [23, 31, 45], which compared outcomes between LPS and LTS.
Morinis et al. [31] compared nine patients with hereditary spherocytosis (HS) undergoing LPS with nine children undergoing LTS over the same period which showed that EBL was greater in the LPS group (188 + 53 vs. 67 + 17 mL; P = 0.02), but transfusion requirements were similar (1/9 vs. 0/9). The LPS group had higher morphine use (4.1 + 0.6 vs. 2.4 + 0.2 days; P = .03), greater time to oral intake (4.4 + 0.7 vs. 2.0 + 0.2 days; P = .01), and longer hospital stay (6.3 + 1.0 vs 2.7 + 0.3 days; P = .005) than the LTS group. There was no significant difference between groups with respect to increase in hemoglobin level. No patient in the LPS group required completion splenectomy after a mean follow-up of 25 months. Groups were similar in sex, age, concomitant cholecystectomy, complication rate and preoperative hospitalizations, transfusions, and spleen size.
Lee et al. [23] compared 22 patients undergoing LTS and 15 patients undergoing LPS and reported that there were significant differences in postoperative complications such as pleural effusion (LTS 9/22 [40.9%], LPS 0/15 [0%], p = 0.005), splenic vein thrombosis (LTS 10/22 [45.5%], LPS 0/15 [0%], p = 0.002), and postoperative hospital stay (LTS 5.4 ± 1.8 days, LPS 4.2 ± 0.8 days, p = 0.027). There were no significant differences between the groups in terms of the operative time (LTS 151.5 ± 98.5 min, LPS 168.6 ± 46.8 min, p = 0.483), intraoperative blood loss (LTS 337.3 ± 188.4 ml, LPS 422.6 ± 187.4 ml, p = 0.185), and transfusion rate (LTS 3/22 [13.6%], LPS 3/15 [20.0%], p = 0.606) As their conclusion, LPS is a feasible, safe surgical procedure in patients with tumorous lesions of the spleen, and it represents an effective approach to reduce postoperative hospital stay and complications.
Li et al. [45] compared 21 patients diagnosed with splenic rupture who underwent LPS and 20 patients diagnosed with splenic rupture who underwent LTS and reported that the counts of platelet (LPS: 147 ± 48 * 109 vs. LS: 282 ± 61 * 109, P = .031) and leukocyte (LPS: 6.7 ± 1.1 * 109 vs. LS: 8.9 ± 1.9 * 109, P = .017) were significantly different. The operation time (LPS: 122.6 ± 17.2 min vs. LS: 110.5 ± 18.7 min, P = 117), intraoperative blood loss (LPS: 174 ± 22 mL vs. LS: 169 ± 29 mL, P = .331), autologous blood transfusion (LPS: 221 ± 36 mL vs. LS: 206 ± 27 mL, P = .078), allogeneic blood transfusion (LPS: 125 ± 25 mL vs. LS: 150 ± 30 mL, P = .878), and conversion to laparotomy (LPS: 0 vs. LS: 0, P = 1.000) were similar. So, they concluded that LPS may benefit emergency patients and does not increase perioperative risks.
Comparison of short-term outcomes between robotic subtotal splenectomy and LSS
Vasilescu et al. [34] compared 32 consecutive subtotal splenectomies by minimal approach in patients with hereditary spherocytosis (22 vs. 10 robotic laparoscopic subtotal splenectomies) and reported that a significant difference was found for the robotic approach regarding blood loss (90 (30–120) ml vs. 35 (15–85) ml, p < 0.05), vascular dissection duration (20 (15–30) min vs. 15 (15–20) min, p < 0.05), and splenic remnant size (10.57 (6.37–17.14) cm3 vs. 8.16 (6.12–11.81) cm3, P < 0.05). They concluded that robotic subtotal splenectomy was comparable to laparoscopy in terms of hospital stay and complication. The main benefits were lower blood loss rate, vascular dissection time, and a better evaluation of the splenic remnant volume.
Discussion
Spleen is an important peripheral immune organ which has many functions such as regulating the circulating blood volume [53, 54], blood filtration, production of a variety of immunoglobulin and opsonins, and regulation of the endocrine system. The primary immunologic function of the spleen is to filter out virulent pathogens and antigens [2]. We all realized that total splenectomy could lead to several severe complications more easily than partial splenectomy such as pulmonary complications, overwhelming post-splenectomy infection (OPSI), and vascular derangements including thromboembolism and subsequent pulmonary hypertension [55]. According to the reports, the most serious complication caused by total splenectomy is OPSI, which can occur in up to 4.4% of the patients with splenectomy and carries a mortality risk of approximately 50–80% [56]. Singer et al. studied that partial splenectomy and using vaccines preoperatively and postoperatively were good ways to prevent OPSI [1] although Ziske et al. [57] reported one case of fatal OPSI occurred 13 years after partial splenectomy for trauma with conservation of about 20% functional splenic parenchyma. The present data indicate that OPSI after partial splenectomy was greatly reduced.
The main technical difficulty for LPS is the risk of intraoperative and/or postoperative bleeding. Difficulties to control bleeding caused by the spleen are mainly related to the specific vascular anatomy. As we know later, the splenic artery is often divided into two or three groups of branches, and in some patients, the number of segments even ranges from three to seven [58]. Therefore, intraoperative ligation of the terminal divisions of the splenic vessels can lead to an ischemic demarcation zone clearly on the spleen surface. This makes the splenic parenchyma resection with less blood loss possible.
The most common indication in this review is splenic cysts. Laparoscopic cyst decapsulation is a safe and feasible option for superficial cysts in some published reports [59]; however, some studies have noted that there was a high recurrence rate of 64% over a mean follow-up of 12 months [60]. Uranues et al. [5] reported that the recurrence developed within a few months after deroofing, and the patients complained more severe symptoms than those they had experienced preoperatively. But for secondary cysts, Mertens et al. [61] concluded that laparoscopic deroofing should be reserved. However, there were five patients with secondary cysts who underwent LPS with well results in the literature 2,22 and 26. The secondary common indications for LPS in this review were splenic hematological diseases. Among them, hereditary spherocytosis was a major indication. But for LPS in this kind of patients, careful consideration on splenic volume remnant was very important. Bader-Meunier et al. demonstrated that leaving 25% of spleen with adequate perfusion was sufficient to preserve splenic function [62]. Growing evidence supports that preservation of 25–30% of the splenic parenchyma allows an appropriate immunologic response to antigen stimulus [5, 63, 64]. So LPS is also a challenging procedure that may be affected by an inappropriate evaluation of the splenic remnant.
According to series article review, the attitude regarding accessory spleens changed during the years. In 2006, Dutta et al. [65] reported that the small accessory spleen was found and left in situ because the overall intent was to leave some spleen intact to retain immunologic function, and its size did not add significantly to the remnant volume. In 2008, Hery et al. [6] reported one patient had an accessory spleen, which was removed during the LPS procedure. In 2012, Vasilescu [34] reported four patients detected with accessory spleens. For the first case, as their experience, they preserved the accessory spleen; afterward, for the last three patients, they choose to remove them to better assess the splenic remnant volume. There are no comparative studies about whether accessory spleen should be removed or not in partial splenectomy.
There are three comparative studies in this review between LPS and LTS. In 2008, Morinis et al. [31] reported that EBL was greater in the LPS group, but transfusion requirements were similar. LPS group had higher morphine use, greater time to oral intake, and longer POS than the LTS group. But these disadvantages may be balanced by retained splenic immune function, and further studies were required to assess long-term splenic function in these patients. So, in 2015, Lee et al. [23] reported that it represents an effective approach to reduce POS and complications for LPS. And there were no significant differences between the groups in terms of the operative time, intraoperative blood loss, and transfusion rate. As their conclusion, LPS was a feasible, safe surgical procedure in patients with tumorous lesions of the spleen. In 2017, Li et al. [45] compared patients diagnosed with splenic rupture and reported that the counts of platelet and leukocyte were less in LPS than LTS with significant difference. And the operation time, intraoperative blood loss, autologous blood transfusion, allogeneic blood transfusion, and conversion to laparotomy were similar. So, they concluded that LPS may benefit emergency patients and does not increase perioperative risks.
In conclusion, there are potential benefits associated with LPS over LTS, and in early series of highly selected patients, LPS appears to be feasible and safe when performed by experienced laparoscopic surgeons. However, there are no future multicenter RCTs or meta-analysis about the comparison between LPS and LTS. So, as a challenging operation, publication bias is a factor that should be considered before we can draw an objective conclusion.
References
Singer DB (1973) Postsplenectomysepsis. In: Anonymous: Perspectives in pediatric pathology, 1edn Year Book Medical Publishers, Chicago, pp 285–311
Wolf HM, Eibl MM, Georgi E et al (1999) Long-term decrease of CD4?CD45RA?T cells and impaired primary immune response after post-traumatic splenectomy. Br J Haematol 107:55–68
Hansen K, Singer DB (2001) Asplenic-hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Pediatr Dev Pathol 4:105–121
Tracy ET, Haas KM, Gentry T, Danko M, Roberts JL, Kurtzberg J, Rice HE (2011) Partial splenectomy but not total splenectomy preserves immunoglobulin M memory B cells in mice. J Pediatr Surg 46:1706–1710
Uranues S, Grossman D, Ludwig L et al (2007) Laparoscopic partial splenectomy. Surg Endosc 21:57–60
Hery G, Becmeur F, Mefat L et al (2008) Laparoscopic partial splenectomy: indications and results of a multicenter retrospective study. Surg Endosc 22:45–49
Ikeda M, Sekimoto M, Takiguchi S et al (2007) Total splenic vein thrombosis after laparoscopic splenectomy: a possible candidate for treatment. Am J Surg 193:21–25
De Schepper AM, Vanhoenacker F, de Beeck BO et al (2005) Vascular pathology of the spleen, part I. Abdom Imaging 30:96–104
Madoff DC, Denys A, Wallace MJ et al (2005) Splenic arterial interventions: anatomy, indications, technical considerations, and potential complications. Radiographics 25(Suppl 1):S191–S211
Uranüs S, Pfeifer J, Schauer C et al (1995) Laparoscopic partial splenic resection. Surg Laparosc Endosc 5:133–136
Poulin EC, Thibault C, DesCôteaux JG et al (1995) Partial laparoscopic splenectomy for trauma: technique and case report. Surg Laparosc Endosc 5:306–310
Hong TH, Lee SK, You YK et al (2010) Single-port laparoscopic partial splenectomy: a case report. Surg Laparosc Endosc Percutan Tech 20:e164–e166
Robert L, Soares JR et al (1998) Laparoscopic complete excision of a splenic epidermoid cyst. J Laparosc Adv Surg Tech 8:237–240
Khelif K, Maassarani F, Dassonville M et al (2006) Laparoscopic partial splenectomy using radiofrequency ablation for nonparasitic splenic cysts in two children. J Laparosc Adv Surg Tech 16:414–417
Gumbs AA, Bouhanna P, Bar-Zakai B et al (2008) Laparoscopic partial splenectomy using radiofrequency ablation. J Laparosc Adv Surg Tech 18:611–613
Jain P, Parelkar S, Shah H et al (2008) Laparoscopic partial splenectomy for splenic epidermoid cyst. J Laparosc Adv Surg Tech 18:899–902
Hua F, Zhang D, Xin Z et al (2011) Laparoscopic partial splenectomy for large splenic epidermoid cyst. Chin Med J 124:1751–1753
Lima M, Reinberg O, Ruggeri G et al (2013) 3D virtual rendering before laparoscopic partial splenectomy in children. J Pediatr Surg 48:1784–1788
Garza-Serna U, Ovalle-Chao C, Martinez D et al (2017) Laparoscopic partial splenectomy for congenital splenic cyst in a pediatric patient: case report and review of literature. Int J Surg Case Rep 33:44–47
Wang X, Wang M, Zhang H et al (2014) Laparoscopic partial splenectomy is safe and effective in patients with focal benign splenic lesion. Surg Endosc 28:3273–3278
Dudi-Venkata NN, Houli N, Weinberg L et al (2014) Laparoscopic partial splenectomy performed by monopolar saline-cooled radiofrequency coagulation. J Laparosc Adv Surg Tech 24:502–505
de la Villeon B, Zarzavadjian Le Bian A, Vuarnesson H et al (2015) Laparoscopic partial splenectomy: a technical tip. Surg Endosc 29:94–99
Lee SH, Lee JS, Yoon YC et al (2015) Role of laparoscopic partial splenectomy for tumorous lesions of the spleen. J Gastrointest Surg 19:1052–1058
Cai HH, An Y, Wu D et al (2016) Laparoscopic partial splenectomy: a preferred method for select patients. J Laparosc Adv Surg Tech 00:1–6
Corcione F, Cuccurullo D, Caiazzo P et al (2003) Laparoscopic partial splenectomy for a splenic pseudocyst. Surg Endosc 17:1850
Smith ST, Scoot DJ, Burdick JS et al (2001) Laparoscopic marsupialization and hemisplenectomy for splenic cysts. J Laparosc Adv Surg Tech 11:243–249
Iimuro Y, Okada T, Sueoka H et al (2013) Laparoscopic management of giant splenic true cyst with partial splenectomy: a case report. Asian J Endosc Surg 6:226–230
Vasilescu C, Tudor S, Popa M et al (2010) Robotic partial splenectomy for hydatid cyst of the spleen. Langenbecks Arch Surg 395:1169–1174
Chinnusamy P, Ahluwalia JS, Palanisamy S et al (2013) Single incision multi-trocar hepatic cyst with partial splenectomy. J Minim Access Surg 9(2):91–94
Quesada R, Poves M, Iglesias E Berjano et al (2016) Laparoscopic partial splenectomy for giant cyst using a radiofrequency-assisted device: a case report. Surg Case Rep 2:82
Morinis J, Dutta S, Blanchette V et al (2008) Laparoscopic partial vs total splenectomy in children with hereditary spherocytosis. J Pediatr Surg 43:1649–1652
Rescorla FJ, West KW, Engum SA et al (2007) Laparoscopic splenic procedures in children: experience in 231 children. Ann Surg 246:683–687
Slater BJ, Chan FP, Davis K et al (2010) Institutional experience with laparoscopic partial splenectomy for hereditary spherocytosis. J Pediatr Surg 45:1682–1686
Vasilescu C, Stanciulea O, Tudor S (2012) Laparoscopic versus robotic subtotal splenectomy in hereditary spherocytosis. Potential advantages and limits of an expensive approach. Surg Endosc 26:2802–2809
Vasilescu C, Stanciulea O, Popa M et al (2008) Subtotal laparoscopic splenectomy and esophagogastric devascularization for the thrombocytopenia because of portal cavernoma—case report. J Pediatr Surg 43:1373–1375
Okano K, Kakinoki K, Suto H et al (2011) Hand-assisted laparoscopic partial splenectomy using an endopath monopolar sealer. Surg Laparosc Endosc Percutan Tech 21:e291–e294
Budzyński A, Demczuk S, Kumiega B et al (2011) Sclerosing angiomatoid nodular transformation of the spleen treated by laparoscopic partial splenectomy. Videosurg Other Miniinvasive Tech 6(4):249–255
Breitenstein S, Scholz T, Schäfer M et al (2006) Laparoscopic partial splenectomy. J Am Coll Surg 204:179–181
Patrzyk M, Glitsch A, Hoene A et al (2011) Laparoscopic partial splenectomy using a detachable clamp with and without partial splenic embolisation. Langenbecks Arch Surg 396:397–402
Benetatos N, Filobbos R, Ammor B (2011) Laparoscopic partial splenectomy for littoral cell angioma. J Surg Case Rep 7:4
Zhang Y, Chen XM, Sun DL et al (2014) Treatment of hemolymphangioma of the spleen by laparoscopic partial splenectomy: a case report. World J Surg Oncol 12:60. https://doi.org/10.1186/1477-7819-12-60
Han XL, Zhao YP, Chen G et al (2015) Laparoscopic partial splenectomy for splenic hemangioma: experience of a single center in six cases. Chin Med J 128:694–697
Wang WD, Lin J, Wu ZQ et al (2015) Partial splenectomy using a laparoscopic bipolar radiofrequency device: a case report. World J Gastroenterol 21(11):3420–3424
Vega EA, Yamashita S, Shin CY et al (2017) Laparoscopic partial splenectomy for unknown primary cancer: a stepwise approach. Ann Surg Oncol 24:1134
Li H, Wei Y, Peng B et al (2017) Feasibility and safety of emergency laparoscopic partial splenectomy. Medicine 96:16
De Greef E et al (2008) Partial laparoscopic splenectomy for splenic abscess because of Salmonella infection: a case report. J Pediatr Surg 43(5):E35–E38
Petroianu A, Cabezas-Andrade MA, Neto RB (2008) Laparoscopic subtotal splenectomy. Surg Laparosc Endosc Percutan Tech 18:94–97
Seshadri PA, Poulin EC, Mamazza J et al (2000) Technique for laparoscopic partial splenectomy. Surg Laparosc Endosc Percutan Tech 10:106–109
De Pastena M, Nikamp MW et al (2018) Laparoscopic hemi-splenectomy. Surg Today 48:735–738
Zheng L, Deng C, Li J et al (2018) Treatment of hemangioma of the spleen by preoperative partial splenic embolization plus laparoscopic partial splenectomy: a case report. Medicine 97:17
Ramia JM, de la Plaza Llamas R, López-Marcano AJ et al (2017) Laparoscopic partial splenectomy for a splenic epidermoid cyst. Cir Esp 95:613–615
Chen J, Shian Y, Longtang X (2018) Laparoscopic partial splenectomy: a safe and feasible treatment for splenic benign lesions. Surg Laparosc Endosc Percutan Tech 28:287–290
Tiron A, Vasilescu C (2008) Role of the spleen in immunity. Immunologic consequences of splenectomy. Chirurgia (Bucur) 103:255–263
Vasilescu C, Stanciulea O, Tudor S et al (2006) Laparoscopic subtotal splenectomy in hereditary spherocytosis: to preserve the upper or the lower pole of the spleen? Surg Endosc 20:748–752
Schilling RF (1997) Spherocytosis, splenectomy, strokes and heart attacks. Lancet 350:1677
Mouttalib S, Rice HE, Snyder D et al (2012) Evaluation of partial and total splenectomy in children with sickle cell disease using an Internet-based registry. Pediatr Blood Cancer 59:100–104
Ziske CG, Muller T (2002) Partial splenectomy: uses of error. Lancet 359:1144
Redmond HP, Redmond JM, Rooney BP et al (1989) Surgical anatomy of the human spleen. Br J Surg 76:198–201
Mackenzie RK, Youngson GG, Mahomed AA (2004) Laparoscopic decapsulation of congenital splenic cysts: a step forward in splenic preservation. J Pediatr Surg 39:88–90
Schier F, Waag KL, Ure B (2007) Laparoscopic unroofing of splenic cysts results in a high rate of recurrences. J Pediatr Surg 42:1860–1863
Mertens J, Penninckx F, DeWever I et al (2007) Long-term outcome after surgical treatment of nonparasitic splenic cysts. Surg Endosc 21:206–208
Bader-Meunier B, Gauthier F, Archambaud F et al (2001) Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood 97:399–403
Van Wyck DB, Witte MH, Witte CL et al (1980) Critical splenic mass for survival from experimental pneumococcemia. J Surg Res 28:14–17
Szczepanik AB, Meissner AJ (2009) Partial splenectomy in the management of nonparasitic splenic cysts. World J Surg 33:852–856. https://doi.org/10.1007/s00268-008-9868-2
Dutta S, Price VE, Blanchette V et al (2006) A laparoscopic approach to partial splenectomy for children with hereditary spherocytosis. Surg Endosc 20:1719–1724
Acknowledgement
Gangshan Liu contributed to literature research and manuscript preparation. Ying Fan contributed to literature research and approved the final version of manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Liu and Dr. Fan have no conflicts of interest or financial ties to disclose. Informed consent was obtained from all individual participants included in this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, G., Fan, Y. Feasibility and Safety of Laparoscopic Partial Splenectomy: A Systematic Review. World J Surg 43, 1505–1518 (2019). https://doi.org/10.1007/s00268-019-04946-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-04946-8