Abstract
Background
The present study aimed to assess the impact of perioperative fluid management on early acute kidney injury (AKI) rate and long-term sequelae in patients undergoing elective colorectal procedures within an enhanced recovery pathway (ERP).
Methods
Retrospective analysis of consecutive patients from a prospectively maintained ERP database (2011–2015) is performed. Pre- and postoperative creatinine levels (within 24 h) were compared according to risk (preoperative creatinine rise ×1.5), injury (×2), failure (×3), loss of kidney function and end-stage kidney disease (RIFLE) criteria. Risk factors for early AKI were identified through logistic regression analysis, and long-term outcome in patients with AKI was assessed.
Results
Out of 7103 patients, 4096 patients (58%) with pre- and postoperative creatinine levels were included. Of these, 104 patients (2.5%) presented postoperative AKI. AKI patients received higher amounts of POD 0 fluids (3.8 ± 2.4 vs. 3.2 ± 2 L, p = 0.01) and had increased postoperative weight gain at POD 2 (6 ± 4.9 vs. 3 ± 2.7 kg, p = 0.007). Independent risk factors for AKI were high ASA score (ASA ≥ 3: OR 1.7; 95% CI 1.1–2.5), prolonged operating time (>180 min: OR 1.9; 95% CI 1.3–2.9) and diabetes mellitus (OR 2.5; 95% CI 1.5–4), while minimally invasive surgery was a protective factor (OR 0.6; 95% CI 0.4–0.9). Five patients (0.1%) developed chronic kidney disease, and two of them needed dialysis after a mean follow-up of 33.7 ± 22.4 months.
Conclusions
Early AKI was very uncommon in the present cohort of colorectal surgery patients treated within an ERP, and long-term sequelae were exceptionally low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Balanced fluid management with a goal of euvolemia is a key item of enhanced recovery pathways (ERP). Fluid overload has been repeatedly associated with adverse postoperative outcomes [1, 2]. While the principle of goal-directed fluid therapy applies for high-risk patients or procedures, the principles of ERP aim for near zero fluid balance in elective surgery, translating into minimal postoperative weight gain [3]. The associated consequence of this principle remains perioperative low urine output, which has been reported to not cause harm [4,5,6].
Recent series, however, have reported worrying rates of acute kidney injury (AKI) of up to 11% in patients undergoing colorectal surgery within ERP [7, 8]. This controversy was rekindled after controversial results of a recent randomized controlled trial found higher rates of AKI in patients undergoing restrictive perioperative fluid management as compared to the liberal fluid group [9].
The present study aimed to assess postoperative AKI rates within 24 h of surgery to study the direct impact of restrictive perioperative fluid management on early AKI rate in the setting of an established ERP. Our secondary aim was to establish the long-term sequelae of those patients suffering from early AKI.
Methods
Patients
All consecutive patients undergoing elective colorectal surgical procedures at Mayo Clinic, Rochester, MN, a tertiary academic teaching facility, between January 1, 2011, and December 31, 2015, were eligible. Data were retrieved from a prospectively maintained institutional electronic enhanced recovery database. All patient-related information was anonymized prior to analysis. This study was approved by the institutional review board.
Demographic information included age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) Physical Status Classification group, presence of diabetes mellitus and preoperative serum creatinine (mg/dL), albumin (g/dL) and hemoglobin levels (g/dL), providing that they were drawn within 90 days of surgery [10]. Surgical information was stratified according to malignant indication, procedure type (colonic, rectal resections, low anterior resections and abdominoperineal resections), ostomy procedures (Hartmann reversals and ostomy closures) and other (small bowel procedures, repair of rectal prolapse and procedures not assignable to either former category), approach (open vs. minimally invasive, including laparoscopic and robotic) and operation duration.
Administered intravenous (IV) fluids including crystalloids, packed red blood cells, albumin and hetastarch (plasma volume expander) were calculated intraoperatively (start to end of anesthesia), at the post-anesthesia care unit (PACU), POD 0 until midnight, POD 1 and POD 2. Further gathered were amounts of ingested oral fluids at POD 0 through POD 2. Weight was assessed preoperatively (within 1 month of surgery) and on postoperative day (POD) 1, 2 and at discharge.
Enhanced recovery pathway
All patients were treated within a comprehensive enhanced recovery pathway (ERP), which was formally implemented in Rochester in 2009 and expanded to include all surgeons as of January 1, 2011. Details regarding this pathway have been extensively described previously, including data on compliance with the pathway [11,12,13,14].
If AKI was identified according to below definition, ERP was adapted as follows:
-
Fluid resuscitation was initiated in concordance with nephrology consult until resolution of AKI on subsequent serum creatinine measurements
-
Potentially nephrotoxic drugs including NSAIDs were discontinued
-
Daily serum creatinine laboratory check until normalization or back to baseline
Outcomes/study endpoints
The primary endpoint was the rate of early AKI within 24 h after surgery, according to risk (preoperative serum creatinine rise ×1.5), injury (×2), failure (×3), loss of kidney function and end-stage kidney disease (RIFLE) criteria, considering a comparison of preoperative baseline creatinine level on POD 1 [15]. Early AKI rate was also assessed using Kidney Disease Improving Global Outcomes (KDIGO) criteria, yielding a postoperative creatinine increase of 0.3 mg/dL [16]. According to institutional practice, serum creatinine (SCr) was measured preoperatively for all patients. Postoperatively it was a standard practice to obtain values on POD 1; however, this standard was executed at a rate of 66%. As a general rule, SCr was not measured in patients without particular previous or ongoing health issues and or those under the age of 50. If on POD 1 AKI was identified or other potential complications in the postoperative course, further measurements were performed on subsequent postoperative days.
If criteria for AKI were fulfilled according to above-mentioned criteria, patient charts were scrutinized through case-by-case analysis to retrieve the following additional information:
-
renal history, considering previous episodes of AKI and previous or present renal disease including preexisting chronic kidney disease (CKD)
-
cardiac history regrouping coronary heart disease (CHD), cardiac failure according to NYHA classification [17] or previous ischemic events (including transient ischemic attacks (TIA) or strokes) and pulmonary embolism
-
pulmonary history, in particular, presence of chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea syndrome
-
Potentially nephrotoxic drugs as current medications by the time of surgery, including angiotensin-converting enzyme (ACE) inhibitors, nonsteroidal anti-inflammatory drugs (NSAID), angiotensin II receptor blockers (ARB) and diuretic treatment.
In addition, in patients with AKI the potential long-term impairment of kidney function, including the need for long-term dialysis, was evaluated. Chronic kidney disease (CKD) was defined according to guidelines of the National Kidney Foundation (estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 sustained for more than 90 days or evidence of structural or functional kidney damage with normal eGFR) [18]. AKI patients were followed until July 31, 2018, or until loss to follow-up or death in order that we could identify potential long-term sequelae (CKD or dialysis).
Secondary endpoints were overall complications other than AKI according to Clavien–Dindo Grades I–V [19], bleeding complications, defined as need for transfusion through POD 0–2, surgical site infections (SSI) needing invasive treatment (either percutaneous, surgical or vacuum dressings), radiologically or clinically confirmed anastomotic leaks, postoperative ileus (defined as need for nasogastric tube reinsertion), reoperation, readmission to Mayo facility and length of hospital stay (procedure until discharge). All complications were assessed in hospital and until 30 days postoperatively (outpatient control visits) and compared between the two groups (patients with early AKI within 24 h after surgery vs. patients without early AKI).
Statistical analysis
Descriptive statistics for categorical variables were reported as frequency (%) and continuous variables as mean (standard deviation) or median (interquartile range IQR). Chi-squared test was used to compare categorical variables. All statistical tests were two-sided, and a level of 0.05 was used to indicate statistical significance. Variables with p values ≤ 0.05 were then entered into a multivariable logistic regression (based on a probit regression model) to provide adjusted estimations of the odds ratio (OR). Data analysis was performed with the Statistical Software for the Social Sciences SPSS Advanced Statistics 22 (IBM Software Group, 200 W. Madison St., Chicago, IL 60606, USA).
Results
Patients
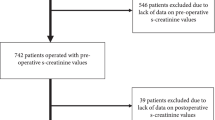
A total of 7103 colorectal procedures were performed over the study period within an ERP. SCr measurements were performed as follows: preoperatively in 6210 patients (87%), postoperatively at POD 1 in 4656 patients (66%) and pre- and postoperatively at POD 1 in 4097 patients (58%). Median elapsed time between preoperative and POD 1 SCr measurements was 4 days (IQR 1–14). Patients with pre- and postoperative creatinine measurements (n = 4097) compared to the excluded remaining cohort (n = 3006) as follows: Significant differences were observed in age (>70 years: 24.4% vs. 16.6%, p < 0.001), ASA score (≥3: 31.5% vs. 20.6%, p < 0.001), surgical duration (>180 min.: 41% vs. 37%, p = 0.001) and diabetes mellitus (10.6% vs. 6%, p < 0.001). No differences were observed regarding minimally invasive approach (37.4% vs. 38.9%, p = 0.198), gender (male: 51.9% vs. 50.1%, p = 0.135) and BMI (>30 kg/m2: 26.6% vs. 24.6%, p = 0.057).
Of the 4097 patients included for further analysis, 104 patients (2.5%) fulfilled RIFLE criteria for AKI: 82 (2%) with preoperative serum creatinine rise ×1.5, 21 (0.5%) with preoperative serum creatinine rise ×2 and one patient (0.003%) with preoperative serum creatinine rise ×3. Using KDIGO criteria, 184 patients (4.5%) of our cohort developed early AKI. Table 1 summarizes demographic and surgical characteristics of both groups (early AKI within 24 h after surgery vs. no early AKI). Upon univariate analysis, AKI patients were older and had higher ASA scores, BMI and incidence of diabetes mellitus and had significantly more open and prolonged procedures (all p < 0.05).
Perioperative fluid management differed between both groups (early AKI within 24 h after surgery vs. no early AKI), with AKI patients receiving higher amounts of total IV fluids at POD 0 [3820 ± 2370 vs. 3200 ± 2040 mL, p = 0.01, of which intraoperative: 3120 ± 2250 vs. 2540 ± 1920 mL (p = 0.011), PACU: 540 ± 560 mL vs. 490 ± 480 mL (p = 0.401), post-PACU until midnight: 160 ± 290 vs. 170 ± 300 mL (p = 0.972)], as well as postoperative fluids at POD 1 and 2, which translated in increased postoperative weight gain at POD 2 (6 ± 4.9 vs. 3 ± 2.7 kg, p = 0.007) and at discharge (2.9 ± 4.5 vs. 1.6 ± 1.1 kg, p = 0.108). AKI patients ingested less oral fluids through POD 0–2, with a significant difference at POD 2 (960 ± 610 vs. 1110 ± 630 mL, p = 0.029). Figure 1 illustrates intravenous and oral fluid balances and evolution of postoperative weight.
Fluid management-related parameters. Comparison of patients with early AKI within 24 h after surgery (n = 104) and patients without early AKI (n = 3993) regarding a intra- and postoperative IV fluid administration, b oral fluid intake through POD 0–2, c weight gain at POD 1, POD 2 and at discharge. AKI acute kidney injury, IV intravenous, POD postoperative day. *Indicates significant values (p < 0.05)
Patients suffering from early AKI had more overall complications as well as individual complications of bleeding, SSI and postoperative ileus. Length of stay was significantly longer in patients with early AKI (8 ± 7 vs. 5 ± 4 days, p = 0.002). Table 2 displays postoperative complication rates of both groups (early AKI within 24 h after surgery vs. no early AKI).
Independent risk factors for early acute kidney injury
Multivariable analysis (Fig. 2) revealed high ASA score [ASA ≥ 3: Odds ratio (OR) 1.67; 95% confidence interval (CI) 1.09–2.54, p = 0.018], prolonged operating time (>180 min: OR 1.91; 95% CI 1.28–2.88, p = 0.02) and diabetes mellitus (OR 2.48; 95% CI 1.53–4.02, p < 0.001) as independent risk factors for early AKI, while minimally invasive surgery was an independent protective factor (OR 0.55; 95% CI 0.35–0.87, p = 0.01).
Multivariable analysis of risk factors for early acute kidney injury. Multivariable analysis of univariate demographic and surgical risk factors (p < 0.05) for early AKI within 24 h after surgery. AKI acute kidney injury, ASA American Society of Anesthesiologists, BMI body mass index. Odds ratio (squares), 95% CI (bars)
Long-term follow-up of patients with early AKI
Mean follow-up time for patients with early AKI was 33.7 ± 22.4 months, with 24 patients (23%) lost to long-term follow-up beyond hospital stay and outpatient control visit (Table 3). In 94 patients (90%), creatinine values normalized (back to baseline level) before discharge of the index hospitalization, while in five patients, creatinine normalized on subsequent measurement within 3 months of surgery.
Five patients (5% of early AKI patients; 0.1% of study cohort) developed or exacerbated chronic kidney disease (CKD). Three of these patients underwent extensive surgery for rectal cancer, and two had preexisting renal disease (one previous kidney transplant with chronic failure and one preexisting diabetic nephropathy). Preoperative creatinine level in these five patients averaged 1.3 ± 0.2 mg/dL. Two patients including the transplant patient needed postoperative dialysis, while the others stabilized their renal dysfunction while attending regular outpatient nephrology control visits during follow-up.
Discussion
The present study revealed a low early postoperative AKI rate of 2.5% (4.5% according to KDIGO criteria) within the first 24 h after surgery in a large cohort of patients undergoing elective colorectal surgery within an established enhanced recovery program. Furthermore, no causal relation of restrictive fluid administration and early postoperative AKI could be established. Over 90% of affected patients recovered to baseline preoperative renal function before discharge, while five patients (0.1% of total cohort) worsened their kidney function over the 3-year follow-up period, ultimately leading to permanent dialysis in two cases (0.04%). Hence, this study contrasts to recent evidence reporting worrying AKI rates after implementation of enhanced recovery care.
A large-scale study examining almost 40,000 patients found that even minor postoperative increases in creatinine levels were associated with adverse outcomes [20]. Both too restrictive management leading to renal hypoperfusion and excessive fluid administration have been associated with devastating effects [21,22,23]. Postoperative AKI strongly correlates with an increased incidence of other postoperative complications and may ultimately lead to CKD [24]. In consequence, caution is warranted in the light of recent studies demonstrating an increase in AKI after ERP implementation [7, 8]. A recent large-scale randomized controlled trial found an 8.7% AKI rate in the restrictive fluid group compared to 5% in the group managed by liberal fluid administration [9]. However, less than half of patients were treated according to enhanced recovery principles [4, 25, 26]. ERPs advocate a near zero fluid balance, embedded within pre- and postoperative liberal oral diet including liquids [4, 27], while goal-directed fluid therapy using invasive monitoring provides useful guidance for high-risk patients or procedures [28,29,30]. Optimal fluid volumes of either approach are, however, not clearly defined in the literature [31]. Fluid administration balances in the present study averaging around 3L at POD 0 were comparable to reports in similar settings [7, 31].
Comparison of AKI rates among studies is difficult considering different definitions and the focus of our study on early postoperative AKI, aiming to specifically assess the impact of stringent fluid management. With 4.5%, the rate using KDIGO criteria was arguably higher than with RIFLE criteria. Whereas RIFLE criteria specifically endorse an abrupt creatinine increase [32], KDIGO implies a 0.3 mg/dL increase within 48 h. Since systematic assessment was performed at 24 h in our institution, RIFLE criteria were used for further analysis. In the study of Hassinger et al. [7], AKI was defined as a rise in serum creatinine ≥1.5 times within 30 days of surgery. In their study, the rate of 12% was similar to the pre-ERAS rate of 13.5%. This is remarkable, since fluid administration decreased considerably from 4.1 to 2.3 L at POD 0 and from 3.6 to 1.3 L at POD 1. The authors found similar risk profiles for AKI as did the present study, including prolonged surgery and open surgery, but also hypertension, dependent functional status and perioperative NSAID use. Our study did not specifically compare these additional items, but the preponderance of antihypertensive drugs and increased ASA score among AKI patients suggest similar patterns.
As a counterpart of the aforementioned studies, Horres et al. [33] found similar rates of AKI using same criteria as the present study when comparing ERP to an adjusted traditional care group, emphasizing the safety of intentional fluid management strategies. Our study compared pre- to early postoperative creatinine levels to identify patients with AKI according to an established definition (RIFLE criteria). Due to this definition, late development of AKI in the setting of other complications was not accounted for, potentially underestimating and underreporting the overall AKI rate, which was 3.8% in former publications of our ERP series before 2011 [13, 34]. However, this approach isolated the impact of perioperative fluid management and the early perioperative aspects of ERP on kidney function prior to the potential confounder of complications. Interestingly, the present study could not confirm a causal effect of restrictive perioperative fluid management and early AKI occurrence. Rather AKI patients received on average higher intraoperative fluids volumes than the remainder of the cohort. Beyond fluid volume, constitutional risk factors such as high ASA score and diabetes mellitus appear to be independent risk factors. Early assessment of renal function at POD 1 allowed for prompt discontinuation of potentially nephrotoxic drugs including NSAIDs and initiation of fluid resuscitation, which may have led to observed encouraging long-term outcomes in this present study.
CKD was very rarely observed, emphasizing the safety of the presently applied ERP, despite scarce use of laboratory parameters. Further, the causative impact of ERP and surgery is uncertain, as preexisting renal problems were present. However, it is important to note that CKD rate was only assessed for patients with early AKI and not for the entire cohort, leading to an inherent risk of underreporting. This approach implies careful identification of at risk patients based on comorbidities, in particular, diabetes, and potentially nephrotoxic preoperative treatments. A proactive selective use of NSAIDs as part of ERP criteria and tailored fluid management strategies in these patients may have contributed to the low AKI rate in the present cohort.
This study has several limitations beyond its retrospective design that need to be addressed. First, the definition of AKI relied on creatinine increase at POD 1 solely, since blood samples were not systematically performed according to ERP protocol. Hence, actual AKI prevalence might be underestimated and underreported, providing that AKI occurred later during the hospital stay. However, this definition was chosen as a pragmatic approach in a high-volume facility with short average hospital stay of 5 days despite a complex surgical practice and considering a cost-conscious environment. The present findings suggest an even more economical use of laboratory evaluation in many patients given the low rate and impact of early postoperative AKI. Second, urine output was not considered for the present analysis, according to recent evidence questioning its utility for perioperative guidance in the first place [4, 6]. Third, a straightforward comparison of preoperative NSAID use in all patients was not possible in the setting of this large retrospective study. Finally, detailed assessment of ERP compliance was not re-performed, assuming that compliance was high according to previous institutional publications [11, 12]. Hence, no cause–effect patterns are claimed through this situation analysis 5 years after implementation of a comprehensive ERP.
Conclusions
The present study, which specifically aimed to assess the impact of restrictive perioperative fluid management in the setting of an established ERP, found a very low rate of early postoperative AKI within 24 h after surgery, and long-term impact of AKI was marginal. Proper patient identification and a standardized, criteria-based application of specific elements of enhanced recovery principles in high-risk patients may be crucial to prevent devastating long-term consequences.
References
Eng OS, Melstrom LG, Carpizo DR (2015) The relationship of perioperative fluid administration to outcomes in colorectal and pancreatic surgery: a review of the literature. J Surg Oncol 111:472–477
Lobo DN, Bostock KA, Neal KR et al (2002) Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 359:1812–1818
Feldheiser A, Aziz O, Baldini G et al (2016) Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 60:289–334
Thiele RH, Raghunathan K, Brudney CS et al (2016) American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med (Lond) 5:24
Gupta R, Gan TJ (2016) Peri-operative fluid management to enhance recovery. Anaesthesia 71(Suppl 1):40–45
Makaryus R, Miller TE, Gan TJ (2018) Current concepts of fluid management in enhanced recovery pathways. Br J Anaesth 120:376–383
Hassinger TE, Turrentine FE, Thiele RH et al (2018) Acute kidney injury in the age of enhanced recovery protocols. Dis Colon Rectum 61:946–954
Marcotte JH, Patel K, Desai R et al (2018) Acute kidney injury following implementation of an enhanced recovery after surgery (ERAS) protocol in colorectal surgery. Int J Colorectal Dis 33:1259–1267
Myles PS, Bellomo R, Corcoran T et al (2018) Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med 378:2263
Ingraham AM, Richards KE, Hall BL, Ko CY (2010) Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg 44:251–267
Larson DW, Lovely JK, Cima RR et al (2014) Outcomes after implementation of a multimodal standard care pathway for laparoscopic colorectal surgery. Br J Surg 101:1023–1030
Lovely JK, Maxson PM, Jacob AK et al (2012) Case-matched series of enhanced versus standard recovery pathway in minimally invasive colorectal surgery. Br J Surg 99:120–126
Khreiss W, Huebner M, Cima RR et al (2014) Improving conventional recovery with enhanced recovery in minimally invasive surgery for rectal cancer. Dis Colon Rectum 57:557–563
Hubner M, Lovely JK, Huebner M et al (2013) Intrathecal analgesia and restrictive perioperative fluid management within enhanced recovery pathway: hemodynamic implications. J Am Coll Surg 216:1124–1134
Koeze J, Keus F, Dieperink W et al (2017) Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol 18:70
Uhlig K, Macleod A, Craig J et al (2006) Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 70:2058–2065
Shafie AA, Tan YP, Ng CH (2018) Systematic review of economic burden of heart failure. Heart Fail Rev 23:131–145
National KF (2015) KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 66:884–930
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Kork F, Balzer F, Spies CD et al (2015) Minor postoperative increases of creatinine are associated with higher mortality and longer hospital length of stay in surgical patients. Anesthesiology 123:1301–1311
Prowle JR, Echeverri JE, Ligabo EV et al (2010) Fluid balance and acute kidney injury. Nat Rev Nephrol 6:107–115
Thacker JK, Mountford WK, Ernst FR et al (2016) Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg 263:502–510
Lobo DN, Stanga Z, Aloysius MM et al (2010) Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three-way crossover study in healthy volunteers. Crit Care Med 38:464–470
Hobson C, Ruchi R, Bihorac A (2017) Perioperative acute kidney injury: risk factors and predictive strategies. Crit Care Clin 33:379–396
Holubar SD, Hedrick T, Gupta R et al (2017) American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond) 6:4
McEvoy MD, Scott MJ, Gordon DB et al (2017) American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: part 1-from the preoperative period to PACU. Perioper Med (Lond) 6:8
Gustafsson UO, Scott MJ, Schwenk W et al (2013) Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg 37:259–284. doi:https://doi.org/10.1007/s00268-012-1772-0
Reisinger KW, Willigers HM, Jansen J et al (2017) Doppler-guided goal-directed fluid therapy does not affect intestinal cell damage but increases global gastrointestinal perfusion in colorectal surgery: a randomized controlled trial. Colorectal Dis 19:1081–1091
Phan TD, D’Souza B, Rattray MJ et al (2014) A randomised controlled trial of fluid restriction compared to oesophageal Doppler-guided goal-directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Anaesth Intensive Care 42:752–760
Som A, Maitra S, Bhattacharjee S, Baidya DK (2017) Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: a meta-analysis and trial sequential analysis of randomized controlled trials. J Anesth 31:66–81
Bundgaard-Nielsen M, Secher NH, Kehlet H (2009) ‘Liberal’ vs. ‘restrictive’ perioperative fluid therapy: a critical assessment of the evidence. Acta Anaesthesiol Scand 53:843–851
Mehta RL, Kellum JA, Shah SV et al (2007) Acute Kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31
Horres CR, Adam MA, Sun Z et al (2017) Enhanced recovery protocols for colorectal surgery and postoperative renal function: a retrospective review. Perioper Med (Lond) 6:13
Huebner M, Hubner M, Cima RR, Larson DW (2014) Timing of complications and length of stay after rectal cancer surgery. J Am Coll Surg 218:914–919
Acknowledgements
Fabian Grass was supported by the Société Académique Vaudoise, Lausanne, Switzerland, and by the SICPA foundation, Lausanne, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grass, F., Lovely, J.K., Crippa, J. et al. Early Acute Kidney Injury Within an Established Enhanced Recovery Pathway: Uncommon and Transitory. World J Surg 43, 1207–1215 (2019). https://doi.org/10.1007/s00268-019-04923-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-04923-1