Abstract
Background
Preoperative simulation of the thoracic duct using magnetic resonance thoracic ductography (MRTD) would enable a safe lymph node dissection near the thoracic duct and the prevention of chylothorax after an esophagectomy. The aim of this study was to determine whether MRTD is useful for preventing injury to the thoracic duct during surgery and for reducing the incidence of chylothorax after surgery.
Methods
We evaluated 130 patients who underwent preoperative MRTD followed by a thoracoscopic esophagectomy for the treatment of thoracic esophageal cancer between August 2014 and April 2017 (MRTD group). These patients were then compared with 160 patients with esophageal cancer who underwent a thoracoscopic esophagectomy without preoperative MRTD (non-MRTD group).
Results
Four patients in the non-MRTD group developed Type IIIB chylothorax (International Consensus on Standardization), while none of the patients in the MRTD group developed Type III chylothorax. Some type of abnormal finding was found during MRTD in 24 patients (18.5%). Among them, 13 patients (10.0%) exhibited abnormal divergence, which was the most frequent finding, followed by 5 patients (3.8%) with window formation and 2 patients (1.5%) with stitch formation.
Conclusions
The present study revealed the frequencies of abnormal findings of the thoracic duct and of patients with false-negative MRTD findings. Injury to the thoracic duct can be avoided through the use of appropriate care during procedures performed in patients with abnormal findings on preoperative MRTD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Confirmation of the thoracic duct (TD) during thoracic esophagectomy for the treatment of thoracic esophageal cancer is very important to enable a safe lymph node dissection near the TD and to prevent chylothorax after an esophagectomy [1]. During its development, the TD first grows as two symmetrical tubes; numerous points of fusion between the two tubes then occur and partly develop or disappear. The lower right part and the upper left part remain and become connected. Finally, the thoracic duct forms as a single tube. The standard route of the TD is upward along the right side of the descending aorta and flowing into the left venous angle. During development, sites that typically disappear sometimes remain, while sites that typically persist sometimes disappear; these processes can result in several anomalies of the TD [2, 3].
Chylothorax caused by injury to the TD can lead to a considerable loss of water and protein, with potentially serious effects on the respiratory and circulatory systems [4, 5]. Moreover, the treatment of chylothorax is very difficult, since the site of TD injury can be difficult to find. At our hospital, the TD is three-dimensionally reconstructed from MRI results obtained before surgery as a part of a preoperative imaging simulation. We believe that preoperative simulation of the TD using magnetic resonance thoracic ductography (MRTD) may enable a safe lymph node dissection near the TD and may help to prevent chylothorax after an esophagectomy.
With the development of endoscopic surgery, the rate of thoracoscopic esophagectomy (TE) for esophageal cancer is now more than 80% at our hospital. The detailed route of the TD can be better visualized by the magnified view available during TE, compared with the situation during a thoracotomy. Therefore, preoperative imaging simulation of the TD is expected to be even more useful than before.
The aim of the present study was to estimate whether MRTD is useful for preventing injuries to the TD during surgery and for reducing the incidence of chylothorax after surgery by providing surgeons with a better awareness of the TD route prior to the start of surgery.
Materials and methods
Patient selection
We evaluated 130 consecutive patients who underwent preoperative MRTD followed by TE for thoracic esophageal cancer at Tokai University Hospital between August 2014 and April 2017 (MRTD group). These patients were then compared with 160 consecutive patients with esophageal cancer who underwent TE before the introduction of MRTD between September 2009 and July 2014 (non-MRTD group).
Surgical procedure
The patients were placed in a prone position. Five trocars were inserted into the right thoracic cavity: three 12-mm trocars and two 5-mm trocars. Only the left lung was ventilated, and a pneumothorax in the right chest was created using 6 mmHg of CO2 gas [6]. Lymph node dissection in the upper mediastinum was first performed, and the TD was identified on the dorsal side of the upper esophagus. If a TD resection was planned, the duct was resected at the level of the cervicothoracic junction. During lymph node dissection in the middle and lower mediastinum, the TD was identified at the level of the aortic arch and was then resected in the lower mediastinum. Generally, TD resection was performed for patients with clinical Stage II or higher advanced esophageal cancer. At the department’s discretion, however, the TD was preserved for patients with severe liver dysfunction (a transaminase level of above 100 IU/L) or a poor nutritive condition (a serum albumin level of below 3.0 g/mL).
Magnetic resonance imaging
The method used for MRTD was previously reported by Nomura, who is a co-author of the present report [7]. MRTD was performed using a 1.5-T clinical scanner (Ingenia; Philips, Best, The Netherland) with a 32-channel torso coil. The MRI scan was performed while the patient was in a supine position without arm elevation. First, sagittal and axial single-shot T2-weighted imaging was performed to roughly identify the location of the thoracic duct. The scanning area for MRTD was then defined as the area from the caudal top of the thoracic duct to the level of the diaphragm. Consequently, the scanning area consisted of an oblique coronal plane, with the cranial portion anteriorly and the caudal portion posteriorly. The actual parameters for MRTD with balanced turbo field echo with extended k-space sampling (bTFEe) were as follows: repetition time/echo time, 5.5/2.8-ms; flip angle, 120 degrees; matrix, 208 × 208; slice thickness, 1.6 mm; turbo factor (number of data samplings per shot), 200; field of view, 300 mm; SENSE factor, 2; number of slices, 100; number of acquisitions, 1.
Statistical analysis
The statistical analysis was performed using SPSS, Ver. 24, software (SPSS Inc., Chicago, IL, USA). The differences in the clinical findings between the MRTD group and the non-MRTD group were analyzed using the Chi-square test and the Student t test. Statistical differences were considered to be significant for P < 0.05.
Results
Patient characteristics in the MRTD group compared with the non-MRTD group
The background data regarding the clinicopathological features in the MRTD group and the non-MRTD group are shown in Table 1. There were no significant differences between the two groups in age, sex, location of tumor, and pathological stage. In general, TD resection was performed for patients with advanced esophageal cancer. However, the TD was preserved at the discretion of the surgeons in patients with severe liver dysfunction or a lower nutritive condition. Moreover, the TD was preserved for two patients (2%) in the MRTD group and for four patients (3%) in the non-MRTD group because of the difficulty in intraoperative localization of TD. The TD was resected in 65 patients (50.0%) in the MRTD group and in 65 patients (40.6%) in the non-MRTD group; no significant difference in the resection rate was seen between the two groups (P = 0.110). Table 2 shows the details of the relationship between TD resection and the pathological stage of cancer in the two groups. There was no difference in the rates of TD resection between the two groups for any stage. Furthermore, no difference was seen in the frequency of lymph node metastasis around the TD, which was found in tissue adjacent to TD during a post-resection examination of the resected tissue, or in tumor invasion to the TD (P = 0.277, P = 0.222, respectively). Nine patients in the non-MRTD group (5.6%) developed chylothorax after surgery (the TD had been resected in eight of these patients), while two patients in the MRTD group (1.5%) developed chylothorax (both in whom the TD had been resected) (P = 0.133). Four patients had Type IIIB chylothorax (International Consensus on Standardization for Complications Associated with Esophagectomy [8]) in the non-MRTD group, while none of the patients in the MRTD group had Type III chylothorax. The TD had been resected in all four patients who developed Type III chylothorax.
Findings of MRTD
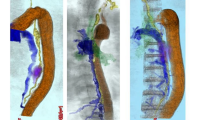
The MRTD findings for all the patients are shown in Table 3. Twenty-four patients (18.5%) had some type of abnormal finding on MRTD. Among them, 13 patients (10.0%) exhibited an abnormal divergence, which was the most frequent finding; the second and third most common findings were window formation (5 patients, 3.8%) and stitch formation (2 patients, 1.5%), respectively. The abnormal divergence was found in the upper area above the aortic arch in eight patients, in the middle and lower areas below the aortic arch in three patients, and throughout both areas in two patients. Window formation and stitch formation were found in the middle and lower areas in all the cases. On the other hand, three cases (2.3%) exhibited abnormal TD findings during surgery, although their preoperative MRTD findings had been normal. Images of the representative abnormal MRTD findings are shown in Fig. 1.
Cases with abnormal divergence of the TD
Case 1
A 63-year-old man was referred to our hospital after complaining of discomfort during swallowing. Endoscopic findings showed a superficial tumor in the middle of the esophagus, and the tumor was pathologically diagnosed as a squamous cell carcinoma after a biopsy. After examination, the clinical staging of the esophageal cancer in this patient was T1N0M0, Stage I (TNM classification, 7th edition), and surgery was planned. Preoperative MRTD showed an abnormal divergence below the aortic arch (Fig. 2a). The TD was located on the dorsal side of the mediastinum (Fig. 2b), and its divergence was found at the left edge of the trachea, where it crossed with the left recurrent laryngeal nerve (Fig. 2c). The TD was not injured during surgery and was successfully preserved.
a MRTD findings for Case 1. An abnormal divergence of the TD at the level of the aortic arch was identified (arrow). b Intraoperative findings at the upper mediastinum. The TD was identified along the left wall of the esophagus (arrow). c A branch of the TD was found along the left wall of the trachea (arrow). The branch crossed the LRN. E esophagus, T trachea, LRN left recurrent laryngeal nerve
Case 2
A 59-year-old woman with dysphagia visited our hospital; after an examination, she was diagnosed as having squamous cell carcinoma of the esophagus (T2N0M0, Stage IB) and surgery was planned. Preoperative MRTD showed a normal TD; however, a section of the TD at the level of the middle mediastinum was unclear (Fig. 3a). A divergent TD was found below the aortic arch, and the right bronchial artery was found between the divergent TD during surgery (Fig. 3b). The TD was resected without injury.
Discussion
In the present study, the rate of abnormal MRTD findings before TE was 18.5%. Abnormal divergence was the most frequent abnormality, and all the abnormal TD routes found during TE were as depicted in MRTD. Moreover, as many of the abnormal divergences were found above the aortic arch, careful attention during lymph node dissection was required in the upper mediastinum.
In terms of TD imaging evaluations, Hayashi et al. first reported the usefulness of MRI in 1999 [9], simulation of the TD using MRI before thoracic surgery was subsequently reported [10], and the usefulness of TD imaging for the diagnosis of chylothorax was also reported [11]. Three-dimensional lymphoscintigraphy was reported as a preoperative imaging simulation of the TD other than MRI [12]. The quality of TD imaging using this modality was higher than that achievable using MRTD. However, MRTD was more useful as a routine evaluation of the TD before surgery because of issues concerning radiation exposure. Adachi et al. reported the anatomical classification of TD. They showed the patterns of TD routes based on the progression of development [13]. One previous report suggested the usefulness of MRI in thoracic surgery for classifying the TD according to the relation between the TD and the spine [14]; however, the patterns and rates of abnormal TD with the potential to influence lymph node dissection during surgery for esophageal cancer have not been previously reported.
The current study showed the patterns, sites, and frequencies of abnormal TD identified during preoperative MRTD with the potential to influence subsequent surgical procedures. MRTD seems to be useful for reducing the incidence of chylothorax. Actually, we were able to avoid careless injury to abnormal TDs present at sites where the normal TD would not be located, such as in Case 1, because the abnormal TD route was successfully identified using MRTD prior to surgery. Recently, TE has been frequently performed for thoracic esophageal cancer, and the detailed route of the TD can be seen more clearly by the magnified view available during TE than by its direct visualization during a thoracotomy. When an abnormal TD finding is observed during MRTD prior to TE, the risk of injury to the TD during TE can be reduced by careful observation of the abnormal TD using a magnified view.
Chylothorax is a rare complication after an esophagectomy for esophageal cancer, with a reported rate of 1–9% [15,16,17,18,19]. As this complication can lead to the loss of water and protein from the body within a short time, it can have serious effects on the performance of the circulatory system and on wound healing during the recovery period after surgery [5, 20, 21]. Moreover, the point of rupture was sometimes difficult to identify when attempting to stop the leakage [22, 23]. In our series, four patients (3%) with chylothorax (Type III according to International Consensus on Standardization for Complications Associated with Esophagectomy) after TE in the era before MRTD performed this procedure, whereas it was not necessary in any of the patients treated after the introduction of preoperative MRTD. In some reports, the rate of chylothorax after TE was 2.4–11.6% [24,25,26]. One report suggested that the rate of chylothorax after TD resection was higher than that after TD preservation [1], and the TD had been resected in 10 of the 11 patients who developed chylothorax after surgery in the present study (91%). We thought that the rationale behind the relationship between TD resection and chylothorax was as follows: Several branches from the TD were failed by TD resection, and the lymphatic flow pressure might increase after TD resection, causing multiple micro-collateral lymphatic routes to rupture and leak lymph. Although there was no difference in the rate of patients who underwent TD resection between the two groups, the rate of patients with chylothorax after surgery tended to be lower in the MRTD group than in the non-MRTD group. This result suggests that preoperative MRTD may help to prevent chylothorax after surgery.
There were three cases (2.3%) whose TD abnormalities were identified during surgery, despite their preoperative MRTD findings being normal. In all of these cases, the abnormal divergence of the TD was observed within the range of the mediastinal lymph node dissection. In Case 2, an unclear area on the preoperative MRTD findings corresponded to the abnormal divergence found during surgery. However, the other two cases did not have any unclear findings, resulting in false-negative MRTD results for these two cases. Surgeons should be aware that a small number of patients may have false-negative MRTD findings, and the clinical features of these patients and procedures for improving the accuracy of MRTD should be investigated in the future.
The limitations of the present study were that it was conducted retrospectively and that a difference in the evaluation periods between the two groups was unavoidable because the period during which the non-MRTD group was treated was prior to the introduction of MRTD. Therefore, a strict comparison of surgical outcomes between the two groups is difficult, although no significant differences were noted. The rates of chylothorax might have been influenced by a selection bias, since the indications for TD resection were based on disease stage and the individual patient’s condition. To prove that MRTD contributes to the prevention of chylothorax, a prospective clinical trial is needed. Additionally, it will be very important to consider cost-effectiveness of preoperative routine MRTD in order to standardize it. We proposed that MRTD might be indicated only in patients scheduled to undergo TD resection. Patients with clinical Stage II or higher advanced esophageal cancer and without liver dysfunction or poor nutrition can be selected, but characteristics of patients most likely to benefit from preoperative MRTD should be investigated in the future.
Although a preventive effect of preoperative MRTD on chylothorax could not be proved statistically in the present study, the frequency of abnormal TD findings and of false-negative MRTD results was identified. Therefore, the present study suggests that TD injury can be avoided by a careful procedure in patients with abnormal findings on preoperative MRTD.
References
Udagawa H, Ueno M, Shinohara H et al (2014) Should lymph nodes along the thoracic duct be dissected routinely in radical esophagectomy? Esophagus 11:204–210
Kotani M (1990) The lymphatics and lymphoreticular tissues in relation to the action of sex hormones. Arch Histol Cytol 53:1–76
Van der Putte SCJ, van Limborgh J (1980) The embryonic development of the main lymphatics in man. Acta Morphol Neerl Scand 18:323–335
Williams KR, Burford TH (1964) The management of chylothorax. Ann Surg 160:131–140
Wemyss-Holden SA, Launois B, Maddern GJ (2001) Management of thoracic dust injuries after oesophagectomy. Br J Surg 88:1442–1448
Ozawa S, Ito E, Kazuno A et al (2013) Thoracoscopic esophagectomy while in a prone position for esophageal cancer: a preceding anterior approach method. Surg Endosc 27:40–47
Nomura T, Niwa T, Kazama T et al (2016) Balanced turbo field echo with extended κ-space sampling: a fast technique for the thoracic ductography. Magn Reson Med Sci 15:405–410
Low DE, Alderson D, Cecconello I et al (2015) International consensus on standardization of data collection for complications associated with esophagectomy. Esophagectomy Complications Consensus Group (ECCG). Ann Surg 262:286–294
Hayashi S, Miyazaki M (1999) Thoracic duct: visualization at nonenhanced MR lymphography—initial experience. Radiology 212:598–600
Okuda I, Udagawa H, Takahashi J et al (2009) Magnetic resonance-thoracic ductography: imaging aid for thoracic surgery and thoracic duct depiction based on embryological consideration. Gen Thorac Cardiovasc Surg 57:640–646
Yu DX, Ma XX, Wang Q et al (2013) Morphological changes of the thoracic duct and accessory lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur Radiol 23:702–711
Takanami K, Ichikawa H, Fukuda H et al (2012) Three-dimensional lymphoscintigraphy using SPECT/CT and 123I-BMIPP for the preoperative detection of anatomical anomalies of the thoracic duct. Clin Nucl Med 37:1047–1051
Adachi B (1953) Der Ductus Thoracicus der Japaner. In: Das Lymphgefasssystem der Japaner, Kenkyu-sha, Tokyo, p 1–83
Okuda I, Udagawa H, Hirata K et al (2011) Depiction of the thoracic duct by magnetic resonance imaging: comparison between magnetic resonance imaging and the anatomical literature. Jpn J Radiol 29:39–45
Dougenis D, Walker WS, Cameron EW et al (1992) Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet 174:501–506
Lai FC, Chen L, Tu YR et al (2011) Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg 91:1770–1774
Mishra PK, Saluja SS, Ramaswamy D et al (2013) Thoracic duct injury following esophagectomy in carcinoma of the esophagus: ligation by the abdominal approach. World J Surg 37:141–146. https://doi.org/10.1007/s00268-012-1811-x
Altorki N, Kent M, Ferrara C et al (2002) Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 236:177–183
Rao DV, Chava SP, Sahni P et al (2004) Thoracic duct injury during esophagectomy: 20 years experience at a tertiary care center in a developing country. Dis Esophagus 17:141–145
Shah RD, Luketich JD, Schuchert MJ et al (2012) Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 93:897–904
Cefolio RJ, Allen MS, Deschamps C et al (1996) Postoperative chylothorax. J Thorac Cardiovasc Surg 112:1361–1366
Orringer MB, Bluett M, Deeb GM (1988) Aggressive treatment of chylothorax complicating trabshiatal esophagectomy without thoracotomy. Surgery 104:720–726
Kaburagi T, Takeuchi H, Oyama T et al (2013) Intraoperative fluorescence lymphography using indocyanine green in a patients with chylothorax after esophagectomy: report of a case. Surg Today 43:206–210
Dexter SPL, Martin IG, McMahonz MJ (1996) Radical thoracoscopic esophagectomy for cancer. Surg Endosc 10:147–151
Luketich JD, Alvelo-Rivera M, Buenaventura PO et al (2003) Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 238:486–495
Smithers BM, Gotley DC, McEwan D et al (2001) Thoracoscopic mobilization of the esophagus: a 6-year experience. Surg Endosc 15:176–182
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Oguma, J., Ozawa, S., Kazuno, A. et al. Clinical Significance of New Magnetic Resonance Thoracic Ductography Before Thoracoscopic Esophagectomy for Esophageal Cancer. World J Surg 42, 1779–1786 (2018). https://doi.org/10.1007/s00268-017-4372-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4372-1