Abstract
Background
Liver and lungs are the two most frequent sites of metastatic spread of colorectal cancer (CRC). Complete resection of liver and/or lung metastases is the only chance of cure, and several studies have reported an improved survival after an aggressive treatment. Nevertheless, CRC liver metastases (CLM) have been recognized as a pejorative factor for patients undergoing pulmonary metastasectomy. We report our experience with patients successively operated on for CRC hepatic and pulmonary metastasis (CPM) and seek to identify prognostic factors.
Methods
All consecutive patients who had resection of CPM and CLM between 2001 and 2014 were enrolled in the study. Clinicopathological and survival data were retrospectively analysed.
Results
Forty-six patients underwent resections of both CLM and CPM. Hepatic resection preceded pulmonary resection in most cases (91.3%). The median intervals between the resection of the primary tumour and the hepatic recurrence and between hepatic and pulmonary recurrences were 12 months [0–72] and 21.5 months [1–84], respectively. The mortality rate following CPM resection was 4.3%. After a median follow-up of 41.5 months [0–126], 35 patients recurred of whom 14 (40%) and 11(31.4%) could benefit from repeated resection of recurrent CLM and CPM, respectively. The median and 5-year overall survivals (OS) were 53 months and 49%, respectively. No prognostic factor was identified.
Conclusion
An aggressive management of CLM and CPM, including repeated resections, may provide a long-term survival comparable to survival of patients with unique metastasectomy. The absence of prognostic factor may reflect the highly selected pattern of the eligible patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) remains the third leading cause of cancer-related mortality in industrial countries, though improvements in chemotherapy and surgical techniques as well as multidisciplinary approaches have radically changed its long-term survival. After curative resection of the primary tumour, metastatic spread occurs in half of patients [1] with liver as the first site of recurrence [2]. Surgical resection is the cornerstone of treatment for CRC liver metastases (CLM), while achieved improvements in chemotherapy have led to consider surgery with perioperative FOLFOX-based treatment as the gold standard [3,4,5]. This strategy provides good results with 5-year survival rates ranging from 39.3 to 57.3% [3, 6]. However, even after multimodal therapy, relapse occurs in 60% within the 2 years following CLM resection [7]. Lung is the second site of recurrence, and pulmonary resection is widely accepted for metastatic spread [8]. In previous series of resected CRC pulmonary metastases (CPM), history of CLM has been considered as a factor of poor prognosis [9] with an increased risk of pulmonary recurrence [10] and a decreased survival [11] when compared to patients operated on for isolated CPM. Nonetheless, survival rates, as high as 50% at 5 years after hepatic and pulmonary resection [12], have been reported in selected patients. In case of hepatic recurrence, repeated CLM resection is considered as an effective treatment with an 5-year overall survival up to 73% [13,14,15]. In the same way, repeated CPM resections have been reported as providing survival consistent with the initial pulmonary metastasectomy [16,17,18]. The results of successive CLM and/or CPM resections remain unclear, as well as those of iterative liver and/or lung resections for recurrent metastatic disease. In this context, we report our institutional experience in the management of patients presenting both CLM and CPM, and particularly those with repeated metastasectomy.
Materials and methods
The Ethical Committee of the French Society of Thoracic and Cardio-Vascular Surgery approved the study and waived the need of individual consent. We retrospectively reviewed medical data of all patients who underwent both pulmonary and hepatic resections for CRC metastases between 2001 and 2014 in the Department of Thoracic Surgery and the Department of Digestive Surgery and Transplantation, Lille University Hospitals, France. Criteria for including patients were: a previous curative treatment of the primary tumour, possibility of macroscopically complete resection of all metastatic sites and sufficient hepatic and pulmonary functions. Hepatic resections were performed as previously reported [19] through midline or right subcostal laparotomy. Pulmonary resections were performed through thoracotomy allowing parenchymal palpation; lung-sparing wedge resection was performed with hilar and mediastinal lymphadenectomy [20] whenever possible; otherwise, a more extended resection was carried out. For patients with bilateral CPM, resections were sequentially performed with a 1-month interval. As a rule, CLM were resected before CPM with the exception of borderline resectable CPM. Pre-operative examinations included chest thin-slice computed tomography (CT), brain CT or magnetic resonance imaging (MRI), an 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) when available, fiberoptic bronchoscopy and pulmonary function test. Administration of perioperative chemotherapy was decided in a multidisciplinary staff meeting, by taking account of the patient’s history and histopathological data. During follow-up, patients were monitored by the referring surgeon and/or oncologist with a physical examination, liver biochemistry assays, carcinoembryogenic antigen (CEA)/carbohydrate antigen 19-9 (CA 19-9) assays and CT of the thorax and the abdomen every 3–6 months. The goal of this follow-up was to allow early detection of recurrence in order to offer repeated resection where possible.
The primary endpoint of this study was the overall survival (OS) defined as the interval from the first lung resection to the date of last follow-up or death. Secondary endpoints were the recurrence patterns and treatments, and prognostic factors.
Continuous variables were expressed as median [range] and categorical data as percentages. Estimates of OS were calculated using the Kaplan–Meier method with log-rank test for comparisons. Statistical significance was set to p < 5%. IBM SPSS (Armonk, New York, USA) v.17 was used for statistical analyses.
Results
From January 2001 to January 2014, among the 109 patients who underwent CPM resection in our institution, 46 patients (42.2%) with history of CLM were included in this study. Patient’s characteristics are summarized in Table 1. There were 23 males (50%). The median age at the time of the first pulmonary resection was 60 years [37–79]. Primary tumour arose from colon in 36 patients (78.3%) and from rectum in the remaining patients. Mostly, the primary tumour was staged T3 in 33 patients (71.7%), with lymph node involvement in 26 patients (56.6%) and synchronous distant metastasis in 21 (45.9%). Synchronous metastases involved the liver in all cases and the lung in one case. CLM resection was carried out before colonic resection in only one patient as a reverse strategy [21]. In the remaining patients, the hepatic resection was performed after colorectal surgery in 37 (80.4%) or during the same procedure in eight (17.4%). Regarding metastases management, CLM resection was performed before CPM in 42 patients (91.3%), while in four (8.7%) CPM were resected first as occurring before CLM. Median interval between CRC and CLM resections, CLM–CPM resection and CRC–CPM resections were 12 months [0; 72], 21.5 months [1; 64] and 36 [2; 98], respectively.
Hepatic procedures
The median interval between the resections of the primary tumour and the CLM was 12 months [0–72]. In the 46 patients, a total of 70 CLM was initially treated, corresponding to a median of 1 CLM per patient [1–5]. The median size of the resected CLM was 2.5 cm [0.5–11]. The most frequent resection was metastasectomy and was performed in 17 cases (34%). Data on the first CLM resection are summarized in Table 2. Data were missing for 11 patients (23.9%) who had undergone hepatic surgery in other centres. Post-operative complications occurred in five patients (10.9%), including lymphorrhea in two and abdominal haematoma, acute respiratory distress syndrome and hepatic failure in one patient each. There was no post-operative death. All but one resection were microscopically complete (R0 resection rate of 97.8%), and 42 patients (91.3%) had perioperative chemotherapy (Table 3).
Pulmonary procedures
Median interval between the resections of the primary tumour and CPM and between CLM and CPM resections was 36 months [2–98] and 21.5 months [1–84], respectively. A total of 91 CPM were initially resected, corresponding to a median of 1.5 CPM per patient [1–4]. The surgery was bilateral and sequentially performed in eight patients (17.4%). Median CPM size was 1.6 cm [0.7–6.7]. The most frequent type of resection was metastasectomy and was performed in 54 cases (76.1%). Data on the first CPM resection are summarized in Table 2. In-hospital mortality rate was 4.3%: one patient died from pyothorax and septic shock and the other one from a Takotsubo cardiomyopathy and multiorgan failure. Seven patients (15.2%) experienced one or more post-operative complications including: atelectasis in two, atrial fibrillation in two, pleural effusion, post-operative pneumonia and left recurrent palsy in one each. On histopathological examination, the rate of CPM R0 resection was 100%, and mediastino-hilar lymph nodes were positive in five (10.9%) patients. Perioperative chemotherapy was administered in 35 (76.1%) patients (Table 3).
Recurrence patterns and survival
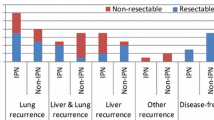
During a median follow-up of 41.5 months [0–126], 35 (76.1%) patients developed relapse of disease within a median delay of 13 months [4.2–21.7]. The two most frequent sites of relapse were lung (N = 24; 68.6%) and liver (N = 22; 47.8%), followed by bones metastases, abdominal lymph nodes, adrenal gland and brain (N = three patients each; 6.5%), peritoneum (N = 2; 4.3%) and diffuse recurrence (N = 1; 2.2%). The metastatic recurrence occurred simultaneously in the liver and the lung in five patients (10.8%).
Among the 22 patients with recurrent metastasis isolated to the liver, 14 patients (63.3%) could benefit from a repeated CLM resection with curative intent including nine (64.3%) metastasectomies and four (28.6%) right hepatectomies (missing data for the last patient); subsequently, six patients had a third hepatectomy and one a fourth. In another patient, a fourth CLM recurrence was treated by stereotactic radiotherapy. The second resection was microscopically complete in 12 patients (R0 rate of 85.7%). Perioperative chemotherapy was administered in 12 patients (85.7%) following the second hepatic resection and in five and one patients after the third and fourth resection, respectively. Patients in whom repeated CLM resection could be performed tended to have a better median OS (38 months, [18.5–57.5]) than patients without re-resection (32 months, [11.8–52.2]). However, the difference was not significant (p = 0.478; Fig. 1).
During follow-up, among the 24 patients with recurrent metastasis isolated to the lung, 11 patients (45.8%) underwent a second CPM resection followed by a third resection in only one case. The second lung resection was achieved by wedge in eight patients (72.7%), segmentectomy, lobectomy and completion pneumonectomy in one patient each (9.1%). Repeated resection was followed by chemotherapy in 81.2% of patients (the only patient who had a third CPM resection did not receive adjuvant chemotherapy). The median OS was significantly longer in patients with redo CPM resection (median OS not reached) than that of patients who could not undergo repeated resections (35 months, [23.3–46.7]); p = 0.008; Fig. 2).
Regarding other sites of recurrence(s), no patient could benefit from curative intent surgery. Median and 5-year OS after the first CPM resection were 53 months [19.9–86] and 49%, respectively (Fig. 3). After exclusion of the two post-operative deaths, the median and 5-year OS increased to 73 months [34–112] and 51%, respectively. After the first CLM resection, median and 5-year OS were 100 months [79.2–120.8] and 63%, respectively. Expectedly, patients with unresectable relapses experienced a significantly lower survival than patients with resectable recurrences or non-recurrent patients, with a median survival of 39 months [29.9–48.1] (p < 0.001). No other prognostic factor was identified from the statistical analysis (Table 4), although subjected to the low number of patients in subgroups. Survival rate was lower in patients with mediastino-hilar involvement, but with no statistical difference.
Discussion
About half of patients with resected CRC will experience liver and/or lung relapse. Although the hypothetical number of CRC patients who will be concerned by bifocal metastatic spread is significant, candidates to concomitant CLM and CPM resections are rare and effectiveness of surgery in that context is not clearly established. Herein, we reported our institutional experience with CPM and CLM resections in the era of modern chemotherapy regimens in order to analyse the survival benefit in this patient population and to seek for selection criteria. Overall, we found an appreciable 5-year OS of 49% following the CPM resection, in association with the frequent possibility of repeated CLM or CPM resection in patients with recurrent metastasis isolated to the liver or lung (re-resection rates of 63.3 and 45.8%, respectively). We could not identify any prognostic factor, suggesting the highly selected pattern of the patients who could be offered concomitant CLM and CPM resections. These results advocate proposing such strategy in patients with a weakly aggressive metastatic disease according to their natural history.
First of all, our study showed that patients with resectable CLM and CPM had an appreciable long-term survival with median and 5-year survival at 53 months and 49% following the CPM resection. Important to note is that only a subset of CLM patients was eligible for resection of CPM, as reflected by the relatively low number of CLM (median of 1.5 per patient and ≤5 in all cases) observed in the current series. Indeed, despite the prognostic value of the CLM number, numerous CLM, e.g. above 10 or more, are not considered as a contra-indication for hepatic resection, provided that a complete R0 resection can be carried out. Contrasting with the current study population, in our previous report on 273 CLM patients, the median number of CLM was only slightly higher, at 2, but up to 17 CLM were resected, and more than 5 in 46 patients [19]. Similarly, number of resected CPM per patient was low with a median of 1 (≤4 in all cases). However, no limit number of CPM has been defined and its low median reflected high selected patients who were candidates to CPM resections. This trend towards a low number of CPM in patients in CLM history was confirmed by previous studies [12, 22, 23].
Isolated repeated CLM [7] or CPM [24] resections are well known and accepted for treatment of resectable metastatic diffusion. However, this aggressive approach remains controversial and PulMiCC trial [25] should bring important responses in the future. Several series [26,27,28] and meta-analysis [29, 30] of CPM resections have been published; however, only a few publications specifically reported patients presenting both CLM and CPM [23, 31] and even more rarely patients with repeated resections in the context of modern oncological and surgical approach [32]. In contrast with early studies [9, 10, 33], a recent meta-analysis [29] could not identify CLM resections as a pejorative factor in patients operated on for CPM. Andres et al. [34] have demonstrated that patients with isolated CLM versus concomitant CLM and CPM resections provided similar 5-year OS rates. Our results were consistent with these previous series showing 5-year survival rates ranging from 39 to 61.3% [9, 11, 33, 35].
Mediastino-hilar lymphadectomy was routinely performed during CPM resection, and several studies [20, 27, 28] demonstrated lymph node involvement had adverse effects on survival. Although incidence of lymph node involvement (10.9%) in our study was consistent with previous publications, we did not identify this parameter as prognostic factor. We can assume the small numbers of patients explained this difference. Currently, there are no data, supporting that lymphadenectomy is required during CPM resection. However, lymphatic involvement can suggest a more aggressive tumoral behaviour and mediastino-hilar lymphadectomy or sampling is recommended [36] to achieve complete tumoral resection and argue systemic treatments.
Additionally in our series, we specifically analysed the pattern of metastatic recurrence(s) after a first CLM and CPM resection and the possibility of iterative curative intent treatment: 35 patients (76.1%) experienced lung and/or liver relapse of whom 14 patients with recurrent CLM (out of 22 patients, 63.3%) could have repeated metastatic resections and 11 of 24 patients (45.8%) with recurrent CPM. To our knowledge, this study is among the first ones that included a high rate of repeated resections of CLM and/or CPM, resulting in a long-term survival comparable to that observed after resection of isolated CPM or CLM. In highly selected patients, redo resections can provide a good OS with an acceptable mortality for both CLM [13,14,15] or CPM [16,17,18] repeated resections. Few previous publications [31] similarly reported outcomes of repeated CPM and CLM resections with good long-term survival rates consistent with our results. In this subset of patients, a favourable tumoral biology may explain relative good results of surgery for such diffuse metastatic disease. Accordingly, no prognostic factor could be identified in the current series, suggesting that a natural selection has occurred upstream, rubbing out the usual prognostic factors such as TNM stage. Although the majority of these patients will develop recurrence, a third or even fourth resection should be proposed whenever possible. Moreover, in our series, very long-term survival beyond 5 years after the first CPM resection was observed in 12 patients (26.1%). Repeated liver and lung resections of CRC metastases could indeed provide prolonged survival that Chua et al. [37] consider as “a curable chronic disease”. Our study had several limitations: data were collected retrospectively, and few patients have been included. However, data were prospectively up dated and patients were closely followed. Furthermore, this series is among the largest series published to date with 46 patients [9,10,11,12, 33, 35, 38,39,40] and more particularly a high rate of redo resection.
In conclusion, an aggressive management including repeated resection of concomitant CLM and CPM may provide an improved long-term survival, similar to that of patients in whom unique metastasectomy was performed, with an acceptable post-operative morbi-mortality rate. A better knowledge in metastatic pathway mechanisms [41,42,43,44] and patients selection may help to define pattern of patients who could benefit from such aggressive treatment.
Abbreviations
- CRC:
-
Colorectal cancer
- CLM:
-
Colorectal liver metastases
- CPM:
-
Colorectal pulmonary metastases
- OS:
-
Overall survival
- CEA:
-
Carcinoma embryonic antigen
- CA 19-9:
-
Carbohydrate antigen 19-9
- 18FDG-PET:
-
18F-fluorodeoxyglucose positron emission tomography
- FOLFOX:
-
Folinic acid–fluorouracil–oxaliplatin
- FOLFIRI:
-
Folinic acid–fluorouracil–irinotecan
- NR:
-
Not reached
References
Berman JM, Cheung RJ, Weinberg DS (2000) Surveillance after colorectal cancer resection. The Lancet 355(9201):395–399
Kobayashi H, Sugihara K (2012) Surveillance and characteristics of recurrence after curative resection for colorectal cancer. In Contemporary Issues in Colorectal Surgical Practice 89
Nordlinger B, Sorbye H, Glimelius B et al (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215
Hebbar M, Pruvot F-R, Romano O et al (2009) Integration of neoadjuvant and adjuvant chemotherapy in patients with resectable liver metastases from colorectal cancer. Cancer Treat Rev 35:668–675. doi:10.1016/j.ctrv.2009.08.005
Portier G, Elias D, Bouche O et al (2006) Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 Trial. J Clin Oncol 24:4976–4982
Scheele J, Stang R, Altendorf-Hofmann A, Paul M (1995) Resection of colorectal liver metastases. World J Surg 19:59–71 doi:10.1007/BF00316981
de Jong MC, Pulitano C, Ribero D et al (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Trans Meet Am Surg Assoc 127:84–92
Pastorino U, Buyse M, Friedel G et al (1997) Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 113:37–49
Zabaleta J, Aguinagalde B, Fuentes MG et al (2011) Survival after lung metastasectomy for colorectal cancer: Importance of previous liver metastasis as a prognostic factor. Eur J Surg Oncol 37:786–790
Landes U, Robert J, Perneger T et al (2010) Predicting survival after pulmonary metastasectomy for colorectal cancer: previous liver metastases matter. BMC Surg 10:17
Hattori N, Kanemitsu Y, Komori K et al (2013) Outcomes after hepatic and pulmonary metastasectomies compared with pulmonary metastasectomy alone in patients with colorectal cancer metastasis to liver and lungs. World J Surg 37:1315–1321 doi:10.1007/s00268-013-1954-4
Sakamoto Y, Sakaguchi Y, Oki E et al (2012) Surgical outcomes after resection of both hepatic and pulmonary metastases from colorectal cancer. World J Surg 36:2708–2713 doi:10.1007/s00268-012-1708-8
Andreou A, Brouquet A, Abdalla EK et al (2011) Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB 13:774–782
Wicherts DA, de Haas RJ, Salloum C et al (2013) Repeat hepatectomy for recurrent colorectal metastases: repeat hepatectomy for recurrent colorectal metastases. Br J Surg 100:808–818
Battula N, Tsapralis D, Mayer D et al (2014) Repeat liver resection for recurrent colorectal metastases: a single-centre, 13-year experience. HPB 16:157–163
Ogata Y, Matono K, Hayashi A et al (2005) Repeat pulmonary resection for isolated recurrent lung metastases yields results comparable to those after first pulmonary resection in colorectal cancer. World J Surg 29:363–368 doi:10.1007/s00268-004-7537-7
Welter S, Jacobs J, Krbek T et al (2007) Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 84:203–210
Chen F, Sakai H, Miyahara R et al (2010) Repeat resection of pulmonary metastasis is beneficial for patients with colorectal carcinoma. World J Surg 34:2373–2378 doi:10.1007/s00268-010-0695-x
Truant S, Séquier C, Leteurtre E et al (2014) Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB 17:176–184
Hamaji M, Cassivi SD, Shen KR et al (2012) Is Lymph node dissection required in pulmonary metastasectomy for colorectal adenocarcinoma? Ann Thorac Surg 94:1796–1800
Mentha G, Majno PE, Andres A et al (2006) Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg 93:872–878
Tsukamoto S, Kinugasa Y, Yamaguchi T, Shiomi A (2014) Survival after resection of liver and lung colorectal metastases in the era of modern multidisciplinary therapy. Int J Colorectal Dis 29:81–87
Matsui T, Kitamura T, Ozawa H et al (2014) Analysis of treatment that includes both hepatic and pulmonary resections for colorectal metastases. Surg Today 44:702–711
Headrick JR, Miller DL, Nagorney DM et al (2001) Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg 71:975–979
Treasure T, Fallowfield L, Lees B (2010) Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 5:S203–S206
Onaitis MW, Petersen RP, Haney JC et al (2009) Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg 87:1684–1688
Okumura S, Kondo H, Tsuboi M et al (1996) Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg 112:867–874
Saito Y, Omiya H, Kohno K et al (2002) Pulmonary metastasectomy for 165 patients with colorectal carcinoma: a prognostic assessment. J Thorac Cardiovasc Surg 124:1007–1013
Gonzalez M, Poncet A, Combescure C et al (2013) Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 20:572–579
Iida T, Nomori H, Shiba M et al (2013) Prognostic factors after pulmonary metastasectomy for colorectal cancer and rationale for determining surgical indications: a retrospective analysis. Ann Surg 257:1059–1064
Neeff H, Hörth W, Makowiec F et al (2009) Outcome after resection of hepatic and pulmonary metastases of colorectal cancer. J Gastrointest Surg 13:1813–1820
Schüle S, Dittmar Y, Knösel T et al (2013) Long-term results and prognostic factors after resection of hepatic and pulmonary metastases of colorectal cancer. Int J Colorectal Dis 28:537–545
Regnard J-F, Grunenwald D, Spaggiari L et al (1998) Surgical treatment of hepatic and pulmonary metastases from colorectal cancers. Ann Thorac Surg 66:214–218
Andres A, Mentha G, Adam R et al (2015) Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br J Surg 102:691–699
Gonzalez M, Robert JH, Halkic N et al (2012) Survival after lung metastasectomy in colorectal cancer patients with previously resected liver metastases. World J Surg 36:386–391. doi:10.1007/s00268-011-1381-3
Reinersman JM, Wigle DA (2016) Lymphadenectomy during pulmonary metastasectomy. Thorac Surg Clin 26:35–40. doi:10.1016/j.thorsurg.2015.09.005
Chua TC, Liauw W, Chu F, Morris DL (2012) Viewing metastatic colorectal cancer as a curable chronic disease. Am J Clin Oncol 35:77–80. doi:10.1097/COC.0b013e3181fe4444
Robinson BJ, Rice TW, Strong SA et al (1999) Is resection of pulmonary and hepatic metastases warranted in patients with colorectal cancer? J Thorac Cardiovasc Surg 117:66–75
Chen F, Shoji T, Sakai H et al (2011) Lung metastasectomy for colorectal carcinoma in patients with a history of hepatic metastasis. Ann Thorac Cardiovasc Surg 17:13–18
Murata S, Moriya Y, Akasu T et al (1998) Resection of both hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer 83:1086–1093
Cejas P, López-Gómez M, Aguayo C et al (2009) KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS ONE 4:e8199
Tie J, Lipton L, Desai J et al (2011) KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res 17:1122–1130
Zong Z, Zhou T, Rao L et al (2016) Musashi2 as a novel predictive biomarker for liver metastasis and poor prognosis in colorectal cancer. Cancer Med 5:623–630
Passiglia F, Bronte G, Bazan V et al (2016) Can KRAS and BRAF mutations limit the benefit of liver resection in metastatic colorectal cancer patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol 99:150–157
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellier, J., De Wolf, J., Hebbar, M. et al. Repeated Resections of Hepatic and Pulmonary Metastases from Colorectal Cancer Provide Long-Term Survival. World J Surg 42, 1171–1179 (2018). https://doi.org/10.1007/s00268-017-4265-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4265-3