Abstract
Background
The surgical Apgar score (SAS) has demonstrated utility in predicting postoperative outcomes in a variety of surgical disciplines. However, there has not been a study validating the utility of the SAS in surgical patients in low-income countries. We conducted a prospective, observational study of patients undergoing laparotomy at a tertiary referral hospital in Rwanda and determined the ability of SAS to predict inpatient major complications and mortality.
Methods
All adult patients undergoing laparotomy in a tertiary referral hospital in Rwanda from October 2014 to January 2015 were included. Data were collected on patient and operative characteristics. SAS was calculated and patients were divided into four SAS categories. Primary outcomes were in-hospital mortality and major complications. Rates and odds of in-hospital mortality and major complications were examined across the four SAS categories. Logistic regression modeling and calculation of c-statistics was used to determine the discriminative ability of SAS.
Results
218 patients underwent laparotomy during the study period. One hundred and forty-three (65.6%) were male, and the median age was 34 years (IQR 27–51 years). The most common diagnosis was intestinal obstruction (97 [44.5%]). A high proportion of patients (170 [78%]) underwent emergency surgery. Thirty-nine (18.3%) patients died, and 61 (28.6%) patients had a major complication. In-hospital mortality occurred in 25 (50%) patients in the high-risk group, 12 (16%) in the moderate-risk group, 2 (3%) in the mild-risk group and there were no deaths in the low-risk group. Major complications occurred in 32 (64%) patients in the high-risk group, 22 (29%) in the moderate-risk group, 7 (11%) in the mild-risk group and there were no complications in the low-risk group. SAS was a good predictor of postoperative mortality (c-statistic 0.79) and major complications (c-statistic 0.75).
Conclusions
SAS can be used to predict in-hospital mortality and major complications after laparotomy in a Rwandan tertiary referral hospital.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparotomy is a common procedure used for a variety of diagnoses with elective and emergent indications. In addition to cesarean section and open fracture treatment, laparotomy is one of the Bellwether procedures [1]. These procedures are so-called because of their ability to serve as proxy for a functioning healthcare system with sufficient material resources and human skills to treat a broad range of essential surgical conditions [1]. Identifying patients at high risk for morbidity and mortality after laparotomy could lead to changes in management, thereby improving outcomes.
In 1953, Virginia Apgar, an anesthesiologist, created a tool to assess newborns [2]. The Apgar test has been used to identify high-risk infants, intervene and ultimately improve neonatal outcomes. Influenced by the effectiveness and simplicity of the Apgar, Gawande and colleagues developed a Surgical Apgar Score (SAS) to provide surgeons with feedback on operative outcomes [3]. The SAS is a 10-point score comprised of 3 intraoperative variables (heart rate, mean arterial pressure and estimated blood loss) [3]. Much like the Apgar, each variable in the SAS is individually scored and summed to create a score ranging from 0 to 10, with lower scores indicating worse outcomes.
Since introduction of the SAS, the tool has been validated in multiple databases and various countries [4–6]. The SAS has utility in various surgical disciplines including orthopedic surgery, colorectal surgery, neurosurgery, gynecology and emergency general surgery [7–13]. Initially, the tool was used to predict 30-day mortality and major complications [3], but now the utility of the SAS has been expanded to predict interventions in surgical step down units, readmissions and intensive care unit (ICU) admissions [13–16].
Most validation studies for the SAS have included patients from high-income countries (HICs) with limited analysis of patients in low-income countries (LICs). Haynes et al. [5] validated the SAS in eight countries, including low- and middle-income countries (LMICs): Tanzania (LIC), India (LMIC), Philippines (LMIC) and Jordan (upper middle-income country). Given the variables used to compute the SAS, this tool could be used in low-resource settings to assist clinicians in risk stratifying postoperative patients.
Rwanda is a LIC in East Africa. It is the most densely populated country in sub-Saharan Africa with a population of 11.34 million [17]. The health system is tiered where patients initially present to a health center before being referred to a district hospital then a referral hospital. More than 90% of the population has some form of health insurance with the majority having government sponsored, community-based health insurance [18]. There is an overall shortage of physicians, including surgeons. Most surgeons live in urban centers, practicing in one of the four tertiary referral hospitals [19, 20]. Postgraduate surgical training has increased with the ultimate goal of staffing a trained surgeon at each district hospital [21].
University Teaching Hospital of Kigali (Centre Hospitalier Universitaire de Kigali, CHUK) is a tertiary referral hospital in Rwanda with a large burden of emergency surgical disease [22]. There are 565 hospitals beds, and 2800 operations performed annually by surgical residents [22]. Laparotomy is a common procedure performed with over 500 cases performed annually, and more than half performed for emergency indications [22, 23]. This study aims to characterize the utility of SAS to identify patients at high risk for morbidity or mortality after laparotomy at a referral hospital in Rwanda. We hypothesize that low SAS scores will be associated with high morbidity and mortality.
Methods
All adult patients undergoing exploratory laparotomy at CHUK between October 2014 and January 2015 were included in this study. Pediatric patients (less than 12 years of age) were excluded as vital signs for infants and children differ from adolescent and adult patients. The SAS is not validated in pediatric patients. Patients were excluded if they had metastatic cancer, unresectable abdominal tumors, non-abdominal operations or poly-trauma patients as these patients were expected to have complex outcomes due to the underlying disease. Emergent gynecological cases conducted by the general surgery teams were included. Elective gynecologic surgeries were excluded as they were conducted in a separate operating facility. Patients were identified by the surgical team and followed through hospitalization. The lead investigator cross-checked operating and recovery room logbooks to ensure patient identification. Data were collected on demographics, past medical and surgical history, diagnosis, operative procedure and complications. The lead investigator followed patients and confirmed details with the operating surgeon to ensure complete data collection.
Operations were classified as emergent or elective. Emergent cases included patients requiring urgent or emergent operation prior to hospital discharge. These patients often presented to the emergency department. Elective operations included patients who presented for a scheduled elective operation.
Diagnoses were classified as intestinal obstruction, appendicitis, perforation, trauma, typhoid, cholecystitis or other. Intestinal obstruction included cases of hernia, intestinal volvulus, intussusception, adhesions, obstructing bands or peritoneal tuberculosis. Appendicitis included patients with appendicular inflammation including early appendicitis, perforated appendicitis or gangrenous appendicitis. Peptic ulcer disease included all patients with gastric or duodenal perforation associated with an ulcer. Trauma included all blunt or penetrating trauma. Typhoid intestinal perforation included all patients with clinical history of typhoid fever and findings of antimesenteric perforation of the ileum at operation. Cholecystitis included patients undergoing operation for gallstone disease.
The SAS score (Table 1) was calculated by summing three intraoperative variables that compromise the score: lowest heart rate, lowest mean arterial pressure (MAP) and estimated blood loss (EBL) [3]. Heart rate and MAP were obtained through anesthesia case logs at the conclusion of the operation. When MAP was not directly recorded, it was calculated from intraoperative recordings of systolic blood pressure (SBP) and diastolic blood pressure (DBP) using the equation: MAP = (SBP + 2 * DBP)/3. EBL was determined based on surgeon estimates. Surgeons were queried at the end of each operation to estimate EBL. The SAS score was stratified into four categories: high risk (SAS 0–4), moderate risk (SAS 5–6), mild risk (SAS 7–8) and low risk (SAS 9–10).
The primary outcomes were in-hospital mortality and major complications. Only in-hospital outcomes were assessed due to resource limitations making 30-day outcomes difficult to assess. Major complications were classified based on American College of Surgeons-National Surgical Quality Improvement Program (ACS NSQIP) modified to the local environment based on treating physician assessment [24]. Complications included: return to operating room, fascial wound dehiscence, evisceration, deep and superficial wound infections, septic shock, deep venous thrombosis, pneumonia, pulmonary embolism, sepsis, acute renal failure, unplanned intubation, coma greater than 24 h, bleeding requiring greater than 4 units of red cell transfusion within 72 h after operation, ventilation greater than 48 h after operation, stroke and cardiac arrest requiring cardiopulmonary resuscitation.
Standard descriptive analysis was employed to examine patient characteristics. Logistic regression calculated the odds of mortality or major complications by SAS category. To test the score’s discriminatory ability, area under the receiver operating characteristic (ROC) curves was generated and c-statistics calculated. Multivariate logistic regression determined independent variables associated with mortality and major complications. Variables included in the multivariate model included age, gender, diagnosis, emergency status and SAS risk category. Data were analyzed using Stata 13.0 (College Station, TX).
The CHUK Ethics Committee, University of Rwanda Institutional Review Board (IRB) and Johns Hopkins IRB approved this study.
Results
Two hundred and eighteen patients met inclusion criteria for the study. Median age was 34 years (IQR: 27–51 years), and 143 (65.6%) patients were male (Table 2). One hundred and eighty-eight (86%) patients reported no past medical history. One hundred and ninety-three (89%) patients reported no past surgical history. Past medical history was classified as chronic conditions, infectious conditions, obstetrics and gynecology or none. Chronic conditions included diabetes mellitus, hypertension, gastritis, peptic ulcer disease, constipation, arthritis or asthma. Infectious conditions included tuberculosis or human immunodeficiency virus. Obstetrics and gynecology included current pregnancy or recent abortion. Past surgical history was classified as general surgery, obstetrics and gynecology orthopedic or none. Prior general surgery operations included exploratory laparotomy and hernia repairs. Prior obstetrics and gynecology operations included caesarean section and hysterectomy. Prior orthopedic operations included fracture fixation.
One hundred and seventy (78%) operations were emergent. Common diagnoses included intestinal obstruction (N = 97, 44%) and appendicitis (N = 30, 14%) (Table 2). Mean operative duration was 102 min (range 30–320 min). There were 39 (18%) deaths and 61 (29%) major complications. Common postoperative complications included deep wound infection (N = 23, 38%) and reoperation (N = 21, 34%) (Table 2). Characteristics of survivors are shown in Table 3. Characteristics of patients with major complications are shown in Table 4.
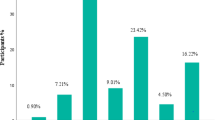
Median SAS was 6 (IQR: 5–7) (Table 2). Fifty (23%) patients had high-risk SAS, 75 (34%) had moderate-risk SAS, 66 (30%) had mild-risk SAS and 27 (10%) had low-risk SAS.
In-hospital mortality increased as SAS decreased. In-hospital mortality occurred in 25 (50%) patients in the high-risk group, 12 (16%) in the moderate-risk group, 2 (3%) in the mild-risk group and there were no deaths in the low-risk group (Table 5). Patients with high-risk SAS had 32 times greater odds of death (95% CI 7.05, 145.24) and patients with moderate-risk SAS had 6.1 greater odds of mortality (95% CI 1.31, 28.34) compared with patients with mild-risk SAS. The discrimination in this model was high with a c-statistic of 0.79 (Fig. 1).
Major complications occurred in 32 (64%) patients in the high-risk category, 22 (29%) in the moderate-risk category, 7 (11%) in the mild-risk category and there were no complications in the low-risk category (Table 5). Patients with a high-risk SAS had 15 times greater odds of major complications (95% CI 5.66, 39.66) and patients with moderate-risk SAS had 3.5 greater odds of major complications (95% CI 1.38, 8.85) compared with patients with mild-risk SAS. The logistic regression model had high discrimination with a c-statistic of 0.75 (Fig. 2).
Combining in-hospital mortality and major complications resulted in similar findings (Table 5). In-hospital mortality or major complications occurred in 33(66%) patients in the high-risk group, 23(30.7%) patients in the moderate-risk group, 7(10.6%) patients in the mild-risk group and no patients in the low-risk group. The odds of in-hospital mortality or major complications were 16 times greater in patients with high-risk SAS (95% CI 6.15, 43.50) and 3.7 times greater in patients with moderate-risk SAS (95% CI 1.48, 9.40) compared to patients with mild-risk SAS.
A multivariate logistic regression model was created using age, gender, diagnosis, emergency status and SAS risk categories (Table 6). Controlling for other variables, in-hospital mortality was associated with SAS (odds ratio (OR) 5.09, 95% CI 2.59, 10.02), age (OR 1.60, 95% CI 1.00, 2.55) and diagnosis (OR 1.32, 95% CI 1.07, 1.63). Major complications were associated with emergency status (OR 4.2, 95% CI 1.16, 15.19), SAS (OR 3.19, 95% CI 1.97, 5.17), diagnosis (OR 1.3, 95% CI 1.09, 1.55) and gender (OR 0.45, 95% CI 0.21, 0.97). Mortality and major complications combined were associated with emergency status (OR 4.32, 95% CI 1.2, 15.61), SAS (OR 3.32, 95% CI 2.04, 5.38) and diagnosis (OR 1.3, 95% CI 1.09, 1.55).
Discussion
This study shows that the SAS can be used in a low-income country with a high burden of emergent surgical conditions to identify patients undergoing exploratory laparotomy who are at high risk of in-hospital mortality and morbidity. A prior study had been conducted in a global patient population, including sites in low- and middle-income countries [5]. However, the previous study was primarily elective operations with few trauma operations and did not separately analyze outcomes from LMICs, making it difficult to compare the findings in a low-income country where emergency operations constitute a large burden of disease [5].
The median SAS score in our cohort was 6, which is lower than the scores reported in previous validation studies [4–6, 10]. Correlating with the lower SAS scores, there is also higher rate of morbidity and mortality in this population, consistent with prior studies [23]. The mortality rate of patients undergoing laparotomy was 18%. This is similar to other low-resource settings where mortality rates range from 15 to 19.5% [25–27]. Prior SAS validation studies reported major complications rates of 9.2–14.1% and mortality rates of 1.4–2.3% [3, 4]. The lower SAS and higher morbidity and mortality rates seen in this patient population are likely attributable to multiple variables. One factor may be the higher proportion of emergency patients in this study. There is a large volume of emergency cases seen at this hospital, whereas previous studies validating SAS have had a lower proportion of emergent cases [3, 4, 6, 10, 15, 23, 28]. The lower SAS in this patient population may be an indication of the overall health of the patients at presentation. The lower SAS suggests that the patients in Rwanda are more critically ill. Finally, an additional reason for lower SAS may be due to differences in intraoperative management of the patients in this setting. The availability and range of anesthetic, analgesia, and vasopressor agents as well as the availability of blood products is limited in low-resource settings, which could lead to differences in intraoperative management. Further studies correlating outcomes with preoperative risk stratification could better elucidate the variations in SAS score. However, this is often difficult in resource-limited settings where a broad range of laboratory studies is not routinely performed or available.
There have been various scoring tools developed to predict postoperative outcomes [29–32]. Many of the variables used in these tools are not routinely collected in low-resource settings. The SAS developed by Gawande and colleagues in 2007 is simple to use at the bedside and effective in predicting postoperative outcomes [3–5]. This study shows that the SAS can be used in a limited-resource setting to predict in-hospital mortality and major morbidity in patients undergoing laparotomy. These findings parallel former validation studies across the different surgical disciplines conducted in HICs [3–13].
The prompt recognition and management of postoperative complications is critical in improving outcomes in surgical patients [33]. A tool such as the SAS can be used to risk stratify patients at risk for postoperative complications. While the SAS has been shown to predict outcomes, few studies have implemented the tool to change clinical management [6]. One study validating SAS at a UK district general hospital found limited opportunities to improve outcomes in patients identified at high risk by SAS [6]. However, the patient population and risk factors in the UK district hospital is likely different than a Rwandan referral hospital. Haddow et al. [34] developed a randomized controlled trial using SAS to guide postoperative care. The incidence of complications was lower in the intervention arm, though this was not statistically significant. Currently, there are no interventions based on SAS in Rwanda, but future studies are being developed that would evaluate interventions based on SAS scores. These include admitting patients with high-risk SAS to a higher level of care such as the ICU or high-dependency unit. As the most common complications found in this study were related to the operative wound (wound infection and wound dehiscence), additional studies will evaluate strategies to improve wound care, preventing infection and wound breakdown in high-risk SAS patients.
While the numbers used to calculate SAS are relatively easy to collect, the data may not always be consistently recorded. EBL is often underestimated by the operating surgeon. However, the SAS EBL categories are sufficiently wide where this is unlikely to have impacted measures. One of the strengths of this study is that accurate data collection was obtained due to constant oversight and supervision from the lead author. It is unclear how accurately these data are collected on a routine basis. The SAS requires frequent monitoring of vital signs intraoperatively. If this is not available, the discriminative ability of the SAS may be altered. Consistent utilization of these data points, however, would encourage more consistent recordings of these values. Local staff may benefit from basic training in collecting data on SAS components to maintain accurate recordings.
Our study has a number of limitations. In under-resourced settings, there is scarce access to routine healthcare and individuals are commonly under-diagnosed or undiagnosed for a variety of medical and surgical illnesses [35–37]. While other studies are able to adjust for these covariates in their analysis, this was not possible due to the limited data available. The study did not collect data on all potential factors associated with adverse outcomes. Prior studies in this hospital have assessed risk factors for morbidity and mortality in various patient populations [23, 38]. In these prior studies, morbidity and mortality were not associated with symptom duration, operating surgeon or anesthesiologist [23]. We were unable to capture data on the cause of death as this is poorly recorded in this setting. The initial Gawande study evaluated 30-day outcomes [3]. However, due to resource limitations, challenges in data collection and outpatient follow-up, all outcomes in this study were in-hospital and clinically diagnosed. Out-of-hospital complications were not captured, and there were limited resources to detect other complications. As such, the complication rate is likely underestimated.
Conclusions
This study demonstrates the ability of SAS to identify patients with postoperative risk after laparotomy in Rwanda. SAS is a tool, using routinely collected data that can be used in limited-resource settings. The findings from this study can be applied to other low-resource settings performing laparotomy with a high burden of emergency operations. Future studies should be developed implementing SAS into clinical practice to guide patient management and resource allocation.
References
Meara JG, Leather AJ, Hagander L et al (2015) Global surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 386:569–624
Apgar V (1953) A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 32:260–267
Gawande AA, Kwaan MR, Regenbogen SE et al (2007) An Apgar score for surgery. J Am Coll Surg 204:201–208
Regenbogen SE, Ehrenfeld JM, Lipsitz SR, et al (2009) Utility of the surgical apgar score: validation in 4119 patients. Arch Surg 144:30–36; discussion 37
Haynes AB, Regenbogen SE, Weiser TG et al (2011) Surgical outcome measurement for a global patient population: validation of the surgical Apgar score in 8 countries. Surgery 149:519–524
Thorn CC, Chan M, Sinha N et al (2012) Utility of the surgical Apgar score in a district general hospital. World J Surg 36:1066–1073. doi:10.1007/s00268-012-1495-2
Wuerz TH, Kent DM, Malchau H et al (2014) A nomogram to predict major complications after hip and knee arthroplasty. J Arthroplasty 29:1457–1462
Wuerz TH, Regenbogen SE, Ehrenfeld JM et al (2011) The surgical Apgar score in hip and knee arthroplasty. Clin Orthop Relat Res 469:1119–1126
Regenbogen SE, Bordeianou L, Hutter MM et al (2010) The intraoperative surgical Apgar score predicts postdischarge complications after colon and rectal resection. Surgery 148:559–566
Ziewacz JE, Davis MC, Lau D et al (2013) Validation of the surgical Apgar score in a neurosurgical patient population. J Neurosurg 118:270–279
Clark RM, Lee MS, Alejandro Rauh-Hain J et al (2015) Surgical Apgar score and prediction of morbidity in women undergoing hysterectomy for malignancy. Gynecol Oncol 136:516–520
Zighelboim I, Kizer N, Taylor NP et al (2010) “Surgical Apgar Score” predicts postoperative complications after cytoreduction for advanced ovarian cancer. Gynecol Oncol 116:370–373
Muthuvel G, Tevis SE, Liepert AE et al (2014) A composite index for predicting readmission following emergency general surgery. J Trauma Acute Care Surg 76:1467–1472
Shum S, Tanzola R, McMullen M et al (2013) How well are prebooked surgical step-down units utilized? J Clin Anesth 25:202–208
Glass NE, Pinna A, Masi A et al (2015) The surgical apgar score predicts postoperative ICU admission. J Gastrointest Surg 19:445–450
Ito T, Abbosh PH, Mehrazin R et al (2015) Surgical Apgar Score predicts an increased risk of major complications and death after renal mass excision. J Urol 193:1918–1922
World Bank Indicators 2015 [December 11, 2015]. Available from: http://data.worldbank.org/indicator
Lu C, Chin B, Lewandowski JL et al (2012) Towards universal health coverage: an evaluation of Rwanda Mutuelles in its first eight years. PLoS ONE 7:e39282
Notrica MR, Evans FM, Knowlton LM et al (2011) Rwandan surgical and anesthesia infrastructure: a survey of district hospitals. World J Surg 35:1770–1780. doi:10.1007/s00268-011-1125-4
Petroze RT, Nzayisenga A, Rusanganwa V et al (2012) Comprehensive national analysis of emergency and essential surgical capacity in Rwanda. Br J Surg 99:436–443
Binagwaho A, Kyamanywa P, Farmer PE et al (2013) The human resources for health program in Rwanda–new partnership. N Engl J Med 369:2054–2059
Rickard JL, Ntakiyiruta G, Chu KM (2015) Identifying gaps in the surgical training curriculum in Rwanda through evaluation of operative activity at a teaching hospital. J Surg Educ. 72:e73–e81
Rickard JL, Ntakiyiruta G, Chu KM (2016) Associations with perioperative mortality rate at a major referral hospital in Rwanda. World J Surg 40:784–790. doi:10.1007/s00268-015-3308-x
ACS NSQIP Participant Use Data File 2015 [September 1, 2015]. Available from: https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use
Memon AA, Siddiqui FG, Abro AH et al (2012) An audit of secondary peritonitis at a tertiary care university hospital of Sindh, Pakistan. World J Emerg Surg 7:6
Majtan P, Neumann J, Kocian P et al (2015) Perspective of patients undergoing surgery for secondary peritonitis. Rozhl Chir 94:199–203
Dawson JL (1963) A study of some factors affecting the mortality rate in diffuse peritonitis. Gut 4:368–372
Melis M, Pinna A, Okochi S et al (2014) Validation of the surgical Apgar score in a veteran population undergoing general surgery. J Am Coll Surg 218:218–225
Copeland GP, Jones D, Walters M (1991) POSSUM: a scoring system for surgical audit. Br J Surg 78:355–360
Khuri SF, Daley J, Henderson W et al (1998) The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 228:491–507
Haga Y, Ikei S, Ogawa M (1999) Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today 29:219–225
Saklad M (1941) Grading of patients for surgical procedures. Anesthesiology 2:281–284
Ghaferi AA, Birkmeyer JD, Dimick JB (2009) Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg 250:1029–1034
Haddow JB, Adwan H, Clark SE et al (2014) Use of the surgical Apgar score to guide postoperative care. Ann R Coll Surg Engl 96:352–358
Mock CN, Donkor P, Gawande A, et al (2015) Essential surgery: key messages of this volume. In: Debas HT, Donkor P, Gawande A, et al (eds) Essential surgery: disease control priorities, 3rd edn., vol 1. World Bank Group, Washington
Weiser TG, Regenbogen SE, Thompson KD et al (2008) An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 372:139–144
Debas HT, Donkor P, Gawande A et al (2015) Essential surgery: disease control priorities, 3rd edn. World Bank Group, Washington
Ndayizeye L, Ngarambe C, Smart B et al (2016) Peritonitis in Rwanda: epidemiology and risk factors for morbidity and mortality. Surgery 160:1645–1656
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest and no financial disclosures to report.
Rights and permissions
About this article
Cite this article
Ngarambe, C., Smart, B.J., Nagarajan, N. et al. Validation of the Surgical Apgar Score After Laparotomy at a Tertiary Referral Hospital in Rwanda. World J Surg 41, 1734–1742 (2017). https://doi.org/10.1007/s00268-017-3951-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3951-5