Abstract
Background
Elevated preoperative serum C-reactive protein (CRP) levels are reportedly associated with a poor prognosis for patients with various types of malignant tumors. However, the impact of postoperative CRP levels on the prognosis of patients with esophageal cancer remains unknown. The present study aims to clarify the prognostic significance of postoperative CRP levels on the survival of patients with esophageal cancer.
Methods
We reviewed the records of consecutive 202 patients with thoracic esophageal squamous cell carcinoma who underwent transthoracic esophagectomy. We measured serum CRP levels on postoperative days (PODs) 1, 2, 3, 5 and 7 and evaluated the relationships between postoperative CRP levels and survival.
Results
The findings of Cox regression analyses suggested that elevated CRP levels on POD 3, 5 and 7 were associated with poor recurrence-free survival (RFS). We divided CRP levels on POD 7 into three tertiles and found that RFS could be clearly stratified, being the poorest (p < 0.001) in the highest tertile (high CRP). The trend was similar even in patients with or without infectious complications and with or without advanced pathological stage. Multivariate analysis showed that pathologically advanced stage (Hazard ratio [HR], 5.14; 95% confidence interval [CI] 2.67–9.87; p < 0.001) and high CRP (HR, 2.27; 95% CI 1.3–3.96; p = 0.004) were independent predictors of RFS.
Conclusion
Postoperative CRP levels could predict the prognosis of patients with esophageal cancer. We propose that the clinical course of postoperative CRP level should be carefully monitored as a predictor of survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite recent improvements in multimodal therapy and perioperative management, the prognosis of patients with esophageal cancer remains poor. A specific predictor of prognosis would be useful for tailoring suitable management strategies to improve the outcomes of patients.
Several biochemical markers such as squamous cell carcinoma antigen [1], carcinoembryonic antigen [2] and CYFRA 21-1 [3] have been investigated, but their sensitivity has not proven fully satisfactory for various stages of esophageal cancer. Whereas C-reactive protein (CRP) is widely regarded as a biochemical marker of a systemic inflammatory response, it has been also indicated that elevated CRP levels are associated with a poor prognosis for patients with various types of malignant tumors [4,5,6,7,8]. Several studies have investigated relationship between pretreatment CRP levels and the prognosis of patients with esophageal cancer [9,10,11,12,13,14,15]. However, the impact of postoperative CRP levels on prognosis in esophageal cancer remains unknown.
A persistent inflammatory state is an important factor in the development of cancer according to several studies [16, 17]. Furthermore, we previously found that excessive surgical stress induces enhanced liver metastasis in rats [18]. Thus, we postulated that postoperatively elevated CRP would reflect a persistent inflammatory state and that postoperative CRP values might serve as a prognostic marker. The present study is aimed to define the prognostic significance of postoperative CRP levels after esophagectomy in patients with esophageal cancer.
Materials and methods
Patients
We reviewed 291 patients who underwent esophageal resection at our institution between January 2003 and December 2011. Among these, 16, 15, 15, 9 and 3 patients were treated by colon interposition, pharyngolaryngoesophagectomy, intrathoracic reconstruction, distal esophagectomy and transhiatal esophagectomy, respectively. Seven patients who were treated by salvage esophagectomy after definitive chemoradiotherapy, 16 with incomplete resection and eight with insufficient postoperative CRP data were excluded from this study. We finally investigated 202 patients who underwent right transthoracic esophagectomy with gastric tube reconstruction and cervical anastomosis using the same approach (three-incision esophagectomy). All tumors were pathologically diagnosed as squamous cell carcinoma. The Institutional Review Board of Hiroshima University approved this study.
Table 1 summarizes the characteristics of the 202 patients (male, n = 177; female, n = 25; mean age, 62.6 years) whose data were analyzed in the present study. All tumors were located in the thoracic segments and staged according to the 7th Edition of the TNM Classification of Malignant Tumors [19]. Postoperative complications developed in 102 patients. Among 62 of those complicated with infections, 41 and 21 were major and minor, respectively.
Treatment modalities
Preoperative therapy was indicated for patients aged <75 years with T2 or higher or lymph node involvement at preoperative clinical evaluations, and whose overall condition was good. Neoadjuvant chemoradiotherapy was the first choice of preoperative therapy at our institution, and neoadjuvant chemotherapy was selected when patients had clinical T1N1 disease or were deemed unsuitable for concomitant radiotherapy and chemotherapy. Patients with clinical T1N0 were immediately treated with surgery, and adjuvant therapy was considered if lymph node involvement was revealed during postoperative pathological evaluation. Therefore, 99 patients received neoadjuvant therapy, and 103 were initially treated by surgery.
All patients underwent at least mediastinal and abdominal (two-field) lymph node dissections. Cervical lymphadenectomy was added when a primary tumor was located in the upper or middle third of the thoracic esophagus, or cervical or upper mediastinal lymph node metastases were clinically evident (three-field lymph node dissection). Subsequently, the gastric tube was lifted via the posterior mediastinal or retrosternal route, and cervical anastomosis was accomplished.
Measurement of serum C-reactive protein
We routinely measured serum CRP levels preoperatively and on postoperative days (PODs) 1, 2, 3, 5 and 7 using a Bio Majesty JCA-BM6070 (Japan Electron Optics Laboratory; Tokyo, Japan) according to the manufacturer’s instructions. Normal values were defined as <1.0 mg/dL. Serum CRP levels were measured at least every seven days until discharge from hospital and at the first outpatient visit, but not routinely thereafter if deemed unnecessary.
Statistical analyses
Data are presented as numbers (%) or as mean ± standard deviation unless otherwise stated. Frequencies were compared using the χ 2 test for categorical variables, and small samples were analyzed using Fisher’s exact test. Postoperative complications were graded according to the Clavien–Dindo classification [20]. Minor complications were defined as grade I and II, and major complications were defined as grades III and higher. Recurrence-free survival (RFS) was defined as elapsed time from the date of surgery until the first event (recurrence or death from any cause) or the last follow-up. The patients were followed up for a median of 41.4 months after surgery.
Correlations between CRP levels and RFS were assessed using Cox proportional hazards models. The duration of RFS was analyzed using the Kaplan–Meier method, and differences in RFS were assessed using the log-rank test. All data were statistically analyzed using SPSS software version 10.5 (SPSS Inc, Chicago, IL, USA).
Results
Postoperative course of CRP levels, complications and survival
Mean CRP values on POD 1, 2, 3, 5 and 7 were 9.85, 19.36, 17.09, 9.77 and 7.89, respectively, indicating a peak on POD 2, followed by a decline (Fig. 1).
Relationship between postoperative CRP levels and survival
We investigated the relationship between RFS and postoperative CRP values using Cox regression analyses. The hazard ratios (HR) of CRP levels on POD 1, 2, 3, 5 and 7 were 0.98 (p = 0.98), 1.03 (p = 0.14), 1.04 (p = 0.03), 1.08 (p = 0.001) and 1.09 (p < 0.001), respectively. Elevated CRP values on POD 3, 5 and 7 were significantly associated with poor RFS. As CRP value on POD 7 (CRP-day 7) had the closest correlation with RFS, we selected this value for subsequent analysis of prognostic impact (Table 2).
Survival based on CRP-day 7
Among 56 patients who died during follow-up, 40 and 14 were due to cancer recurrence and other diseases, respectively, and two were treatment related.
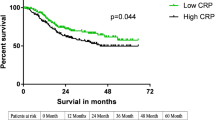
The patients were divided into groups with low, middle and high CRP-day 7 values of 0.67–5.16 (n = 68), 5.21–8.60 (n = 68) and 8.61–36.1 (n = 66), respectively. Recurrence-free survival was clearly stratified among these groups (Fig. 2a). The 5-year survival rates were 48.5, 69.4 and 77.8% for patients in the highest (high CRP), middle (middle CRP) and lowest (low CRP) tertiles, respectively. Survival rates significantly differed between the groups with high and low CRP (p < 0.001) and with high and middle CRP (p = 0.01) values, but not between those with middle and low CRP values (p = 0.24). The RFS was significantly poorer in the group with high than with middle and low CRP values (p < 0.001; Fig. 2b).
Recurrence-free survival (RFS) curves of patients according to C-reactive protein values on postoperative day 7 (CRP-day7). a Five-year RFS rates are 77.8, 69.4 and 48.5% for patients in the lowest (low CRP), middle (middle CRP) and highest (high CRP), tertiles, respectively. b Five-year RFS rates are 73.6 and 48.5% for patients with combined middle and low CRP values and with high CRP values, respectively (p < 0.001)
Comparison of clinical parameters between high and middle and low CRP groups
The ratio of male patients was higher in the group with high, than with combined middle and low CRP values (64/66 [97.0%] vs. 113/136 [87.1%], p = 0.005). Moreover, infectious complications were more frequent in the group with high, than with combined middle and low CRP values (32/66 [48.4%] vs. 30/136 [22.1%], p < 0.001). The mean values for preoperative CRP, albumin, surgical duration and blood loss were higher in the group with high CRP than in the other two groups combined, but the differences did not reach significance (0.51 ± 1.27 vs. 0.35 ± 0.92 mg/dL, p = 0.31; 4.12 ± 0.51 vs. 4.05 ± 0.46 mg/dL, p = 0.36; 421 ± 80.3 vs. 400 ± 75.4 min, p = 0.09; 612 ± 500 vs. 546 ± 405 g, p = 0.35, respectively). The proportion of advanced cancer according to pathological stage was similar between the high versus combined middle and low CRP groups (31/66 [47.0%] vs. 63/136 [46.3%], p = 0.67).
We investigated the effects of infectious complications and pathological stage on the relationship between them using a subgroup analyses based on CRP-day 7 and RFS. Five-year RFS rates were 73.3 and 58.1% for patients without infectious complications who had middle and low combined, and high CRP values, respectively (p = 0.04) (Fig. 3a), while these rates were 80.0 and 36.7%, respectively, for patients with minor infectious complications (p = 0.24) (Fig. 3b) and 80.0 and 34.9%, respectively, for those with major infectious complications (p = 0.01) (Fig. 3c). Furthermore, RFS was also poorer in the high CRP group regardless of pathological stage. Five-year RFS rates are 90.8 and 64.2% for patients in subgroup with pathological stages 0 and I, and with combined middle and low CRP values and high CRP values, respectively (p < 0.001) (Fig. 3d), while these rates are 53.9 and 30.6% for patients in subgroup with pathological stages II, III and IV (p = 0.01) (Fig. 3e).
Recurrence-free survival (RFS) curves of patients according to C-reactive protein values on postoperative day 7 (CRP-day7). a Five-year RFS rates are 73.3 and 58.1% for patients without infectious complications and with combined middle and low CRP values and high CRP values, respectively (p = 0.04). b Five-year RFS rates are 80.0 and 36.7% for patients with minor infectious complications and with combined middle and low CRP values and high CRP values, respectively (p = 0.24). c Five-year RFS rates are 80.0 and 34.9% for patients with major infectious complications and with combined middle and low CRP values and high CRP values, respectively (p = 0.01). d Five-year RFS rates are 90.8 and 64.2% for patients with pathological stages 0 and I, and with combined middle and low CRP values and high CRP values, respectively (p < 0.001). e Five-year RFS rates are 53.9 and 30.6% for patients with pathological stages II, III and IV and with combined middle and low CRP values and high CRP values, respectively (p = 0.01)
Perioperative factors associated with recurrence-free survival
We examined the relationships between several perioperative factors and RFS to identify independent predictors for RFS (Table 3). Univariate Cox regression analysis selected pathological stage (HR, 4.12; 95% CI 2.37–7.15; p < 0.001), and CRP-day 7 (HR, 2.45; 95% CI 1.50–3.99; p < 0.001) as being significantly associated with RFS. Multivariate analysis including sex, infectious complications, and preoperative albumin and CRP values also selected pathological advanced stage (HR, 5.14; 95% CI 2.67–9.87; p < 0.001) and high CRP (HR, 2.27; 95% CI 1.3–3.96; p = 0.004) as independent covariates for RFS.
Survival impact of persistent CRP in High CRP group
Among 66 patients with high CRP group, 50 patients (75.8%) were evaluated serum CRP level at 1 month after surgery (POD 27-31). Five-year RFS rates for patients with persistently high (>1.0 mg/dL; N = 27) or normal (CRP ≤ 1.0 mg/dL; N = 23) CRP values were 25.7 and 68.6%, respectively, indicating significantly poorer RFS for patients with persistently high CRP values (p = 0.005; Fig. 4).
Discussion
We aimed to identify the prognostic impact of postoperative CRP values in patients with esophageal cancer. Several studies have investigated the relationship between CRP values and the prognosis of patients with esophageal cancer. Reports indicate that high CRP levels are associated with a poor prognosis for patients with esophageal cancer treated by esophagectomy [9,10,11,12], radiotherapy [13], chemoradiotherapy [14] and neoadjuvant therapy followed by esophagectomy [15]. However, all of these reports investigated pretreatment CRP levels. To our knowledge, this is the first report about the relationship between postoperative CRP values and the prognosis of patients with esophageal cancer.
The liver is the general site of CRP synthesis, which is regulated by cytokines such as interleukin-1, tumor necrosis factor and mostly interleukin-6 (IL-6). These cytokines are synthesized by monocytes, macrophages, lymphocytes, fibroblasts and endothelial cells at postoperative surgical sites, and elevate serum CRP levels [21, 22].
Cox regression analyses significantly associated CRP-days 3, 5 and 7 with RFS and CRP-day 7 were most useful for stratifying for RFS in our cohort. We collected the patients with same surgical procedure to unify the surgical invasion. However, some confounders still could have affected this result. For example, more surgical stress might be imposed by surgery for advanced, than for early stage cancer, because surgical duration would be extended and more blood would be lost. Moreover, postoperative infectious complications could cause CRP to increase. We attempted to address this issue using a sub-analysis, the results of which uncovered a significant impact of CRP-day 7 upon survival regardless of infectious complications and pathological stage. Furthermore, multivariate analysis identified CRP-day 7 as an independent predictive factor for RFS. These results suggest that postoperative CRP levels have prognostic significance for patients with esophageal cancer treated by esophagectomy.
Postoperative CRP levels affect the prognosis of patients with some other types of malignant tumors. Shiba et al. [23] found that postoperative peak CRP predicted the overall survival of patients with hepatocellular carcinoma. Others have also shown that postoperative CRP levels have prognostic impact for patients with renal cell carcinoma [24, 25]. The reason was thought that this type of carcinoma generates IL-6 and CRP, and an increase postoperative CRP levels would suggest occult residual cancer. The reason why postoperative CRP level is correlated with prognosis in patients with esophageal cancer remains unknown. Like renal cell carcinoma, some types of esophageal squamous cell carcinoma generate IL-6 [26] and CRP [27, 28], which might reflect residual tumors when postoperative CRP is not normalized.
We propose another reason that could explain how postoperative CRP levels affect prognosis. Postoperative CRP levels might differ due to individual anti-inflammatory and immune responses [29, 30]. Indeed, a gene associated with inflammation is activated in obese patients and carriers of hepatitis virus [31, 32]. Elevated postoperative CRP levels are thought to indicate persistent inflammation, and which could cause the development of cancer, and IL-6 that mainly regulates CRP synthesis, is thought to play important roles through activities such as stimulating of angiogenesis [33] and inhibiting cancer from undergoing apoptosis [34]. Furthermore, CRP also has several immunological functions [35]. These findings suggest that a persistent inflammatory state in the presence of postoperative occult residual cancer would be more likely to cause cancer development and recurrence and thus a poor prognosis. Therefore, this could be the most reasonable and convincing explanation for the finding that CRP-day 7 most significantly correlated with RFS in the present study.
Figure 4 shows extremely poor survival among patients with persistently high CRP values, indicating that a long-term inflammatory state caused poor survival. Because esophagectomy is highly invasive, several investigators have applied drugs to suppress excessive reaction to surgical stress and to reduce postoperative complications. Indeed, corticosteroids [36] and sivelestat, a selective neutrophil elastase inhibitor [37], have reduced postoperative surgical stress reaction after esophagectomy. The present and these findings with this result suggest that such drugs might also help to suppress the progression of occult residual cancer. We previously showed that sivelestat suppresses the growth of gastric cancer cells by inhibiting the release of TGF-α stimulated by neutrophil elastase [38]. Eicosapentaenoic acid reportedly modifies the activity of cytokines such as IL-6 and inhibits the proliferation of an esophageal cancer cell line [39].
The present study was limited by the retrospective design. Our patients had various stages of esophageal cancer and were therefore treated according to various policies. Several perioperative factors affect postoperative CRP values. However, we selected the patient population and used subgroups and multivariate analysis to avoid the influence of some confounders. Although these strategies allowed comparisons between patients who had been exposed to similar degrees of surgical stress, the influence of perioperative factors upon postoperative CRP levels might be difficult to completely exclude. Therefore, a validation study is needed to confirm our results in large cohorts who are with similar characteristics.
In conclusion, our findings suggest that elevated postoperative CRP levels are associated with poor survival in patients with esophageal cancer. We therefore propose that the clinical course of postoperative CRP should be carefully monitored as a predictor of survival.
References
Shimada H, Nabeya Y, Okazumi S et al (2003) Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery 133:486–494
Munck-Wikland E, Kuylenstierna R, Wahren B et al (1988) Tumor markers carcinoembryonic antigen, CA 50, and CA 19-9 and squamous cell carcinoma of the esophagus. Pretreatment screening. Cancer 62:2281–2286
Brockmann JG, St Nottberg H, Glodny B et al (2000) CYFRA 21-1 serum analysis in patients with esophageal cancer. Clin Cancer Res 6:4249–4252
Alifano M, Falcoz PE, Seegers V et al (2011) Preresection serum C-reactive protein measurement and survival among patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 142:1161–1167
Baba H, Kuwabara K, Ishiguro T et al (2013) C-reactive protein as a significant prognostic factor for stage IV gastric cancer patients. Anticancer Res 33:5591–5595
Jang JW, Oh BS, Kwon JH et al (2012) Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine 60:686–693
Szkandera J, Stotz M, Absenger G et al (2014) Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer 110:183–188
Hu Q, Gou Y, Sun C et al (2014) The prognostic value of C-reactive protein in renal cell carcinoma: a systematic review and meta-analysis. Urol Oncol 32(50):e1–e8
Nozoe T, Saeki H, Sugimachi K (2001) Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg 182:197–201
Ikeda M, Natsugoe S, Ueno S et al (2003) Significant host- and tumor-related factors for predicting prognosis in patients with esophageal carcinoma. Ann Surg 238:197–202
Shimada H, Nabeya Y, Okazumi S et al (2003) Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol 83:248–252
Gockel I, Dirksen K, Messow CM et al (2006) Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol 12:3746–3750
Wang CY, Hsieh MJ, Chiu YC et al (2009) Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol 92:270–275
Guillem P, Triboulet JP (2005) Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus 18:146–150
Zingg U, Forberger J, Rajcic B et al (2010) Association of C-reactive protein levels and long-term survival after neoadjuvant therapy and esophagectomy for esophageal cancer. J Gastrointest Surg 14:462–469
Ben-Baruch A (2006) Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol 16:38–52
Keibel A, Singh V, Sharma MC (2009) Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des 15:1949–1955
Hirai T, Yoshimoto A, Iwata T et al (1997) Enhancing effect of thoraco-laparotomy on liver metastasis and the role played by active oxygens in its mechanism. Surg Today 27:1040–1045
Sobin LH, Gospodarowicz MK, Wittekind CH (2009) TNM classification of malignant tumours, 7th edn. Wiley, Hoboken
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Aarden LA, De Groot ER, Schaap OL et al (1987) Production of hybridoma growth factor by human monocytes. Eur J Immunol 17:1411–1416
Sironi M, Breviario F, Proserpio P et al (1989) IL-1 stimulates IL-6 production in endothelial cells. J Immunol 142:549–553
Shiba H, Furukawa K, Fujiwara Y et al (2013) Postoperative peak serum C-reactive protein predicts outcome of hepatic resection for hepatocellular carcinoma. Anticancer Res 33:705–709
Johnson TV, Abbasi A, Owen-Smith A et al (2010) Postoperative better than preoperative C-reactive protein at predicting outcome after potentially curative nephrectomy for renal cell carcinoma. Urology 76(766):e1–e5
Ito K, Yoshii H, Sato A et al (2011) Impact of postoperative C-reactive protein level on recurrence and prognosis in patients with N0M0 clear cell renal cell carcinoma. J Urol 186:430–435
Chen MF, Chen PT, Lu MS et al (2013) IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer. doi:10.1186/1476-4598-12-26
Nozoe T, Korenaga D, Futatsugi M et al (2003) Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus—significance as a tumor marker. Cancer Lett 192:89–95
Nakatsu T, Motoyama S, Maruyama K et al (2012) Tumoral CRP expression in thoracic esophageal squamous cell cancers is associated with poor outcomes. Surg Today 42:652–658
Roth-Isigkeit A, Hasselbach L, Ocklitz E et al (2001) Inter-individual differences in cytokine release in patients undergoing cardiac surgery with cardiopulmonary bypass. Clin Exp Immunol 125:80–88
Misoph M, Babin-Ebell J (1997) Interindividual variations in cytokine levels following cardiopulmonary bypass. Heart Vessels 12:119–127
Lee Y, Park US, Choi I et al (1998) Human interleukin 6 gene is activated by hepatitis B virus-X protein in human hepatoma cells. Clin Cancer Res 4:1711–1717
Lee YH, Nair S, Rousseau E et al (2005) Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia 48:1776–1783
Nagasaki T, Hara M, Nakanishi H et al (2014) Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer 110:469–478
Gado K, Domjan G, Hegyesi H et al (2000) Role of interleukin-6 in the pathogenesis of multiple myeloma. Cell Biol Int 24:195–209
Black S, Kushner I, Samols D (2004) C-reactive protein: minireview. J Biol Chem 279:48487–48490
Sato N, Koeda K, Ikeda K et al (2002) Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg 236:184–190
Kawahara Y, Ninomiya I, Fujimura T et al (2010) Prospective randomized controlled study on the effects of perioperative administration of a neutrophil elastase inhibitor to patients undergoing video-assisted thoracoscopic surgery for thoracic esophageal cancer. Dis Esophagus 23:329–339
Wada Y, Yoshida K, Hihara J et al (2006) Sivelestat, a specific neutrophil elastase inhibitor, suppresses the growth of gastric carcinoma cells by preventing the release of transforming growth factor-alpha. Cancer Sci 97:1037–1043
Kubota H, Matsumoto H, Higashida M et al (2013) Eicosapentaenoic acid modifies cytokine activity and inhibits cell proliferation in an oesophageal cancer cell line. Anticancer Res 33:4319–4324
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Ibuki, Y., Hamai, Y., Hihara, J. et al. Role of Postoperative C-Reactive Protein Levels in Predicting Prognosis After Surgical Treatment of Esophageal Cancer. World J Surg 41, 1558–1565 (2017). https://doi.org/10.1007/s00268-017-3900-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3900-3