Abstract
Background
Techniques for accurately delineating the tumor bed after breast-conserving surgery (BCS) can be challenging. As a result, the accuracy, and efficiency of radiation treatment (RT) planning can be negatively impacted. Surgically placed clips or the post-surgical seroma are commonly used to determine target volume; however, these methods can lead to a high degree of uncertainty and variability. A novel 3-dimensional bioabsorbable marker was used during BCS and assessed for its impact on RT planning.
Methods
One hundred and ten implants were sutured to the margins of the tumor bed excision site in 108 patients undergoing BCS. Routine CT imaging of the breast tissue was performed for RT planning, and the marker was assessed for visibility and utility in target delineation. RT regimens, target volumes and associated treatment costs were analyzed.
Results
In all patients, the marker was easily visible and in 95.7 % of cases, it proved useful for RT planning. 36.8 % of patients received conventional whole breast irradiation plus boost, 56.6 % received hypo-fractionation plus boost, and 6.6 % received accelerated partial breast irradiation. A shift toward increased use of hypo-fractionated regimens was noted over the three year period of this study. There were no device-related complications or cancer recurrences in this group of patients.
Conclusions
This study demonstrated the use of a novel 3-dimensional marker as a safe and effective method for delineating the tumor bed with a significant utility for RT planning. With routine use of the device, an increased use of hypofractionation with a resultant 25 % cost savings was noted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast conservation surgery (BCS) exemplifies the height of progress in the surgical management of breast cancer. In most cases, post-operative radiation therapy (RT) is an essential component of treatment in order to reduce local recurrence rates, and achieve survival rates consistent with mastectomy [1–4]. In regard to preventing local recurrence, the tumor excision site is at highest risk, with 80 % of local cancer recurrences occurring at the surgical excision site of the tumor bed [5–7]. This site-specific nature of recurrence risk is particularly important in patients with certain tumor characteristics (e.g., DCIS with comedonecrosis), and those patients with close surgical margins (less than a few millimeters). Thus, in the treatment of breast and other cancers, surgeons strive to identify the tumor bed in order to assist with targeting of post-operative RT and follow-up. Given the recent guidelines regarding “no tumor on ink”, set forth by the Society of Surgical Oncology (SSO) and the American Society for Therapeutic Radiation Oncology (ASTRO), greater precision in radiation targeting to the tumor bed is a timely topic of concern [8].
The majority of BCS patients today receive whole breast irradiation plus a boost, to the tumor bed. However, there is widespread interest in using more advanced techniques, such as hypo-fractionated and/or accelerated treatment regimens [9–18]. These methods are attractive for many reasons, most notably decreased time for patients to complete their treatment (thereby reducing the arduous nature of a six-week, daily course of radiation) and the opportunity for significant cost savings. One factor that has hindered adoption of these techniques is the difficulty in accurately targeting the surgical tumor bed in order to maintain tight parameters and smaller treatment volumes. In many cases, uncertainty or ambiguity in the ability to target the tumor bed leads to target volumes that are simply too large to treat in an accelerated manner, without the potential to cause an increase in toxicities [19–30]. While several methods for targeting the tumor bed are currently used, none of them produce an easily visible, standardized target that is reliable during the post-operative period of healing and the associated changes to the breast.
Currently, the region targeted for radiation treatment is defined by individual surgical clips placed by the surgeon or the presence of post-surgical tissue changes and seroma. Both of these methods have notable limitations that introduce concerns when determining target volumes [31]. To address these limitations, a surgical tissue marker has been introduced that can be sutured directly to the surgical site as an indicator, thereby providing a visual guide for RT planning. Theoretically, use of this marker could serve to provide an enhanced method of communication between the surgeon and radiation oncologist to assist planning and targeting [32–34]. In order to assess the utility of this surgically implanted marker, we tracked the impact of its ability to help visualize the tumor excision site and its potential impact on RT planning.
Materials and methods
Pre-operative
Following informed consent, 108 consecutive patients were prospectively selected for implantation with a 3-D “volumetric” implantable marker (BioZorb™Tissue Marker, Focal Therapeutics, Aliso Viejo, CA) at the time of BCS (partial mastectomy/lumpectomy). The marker is composed of a semi-rigid, bioabsorbable spiral framework made of polylactic acid (PLA) with a fixed array of 6 titanium clips embedded within the spiral. All patients received a comprehensive pre-operative workup that included mammography, ultrasound, MR imaging (where appropriate), minimally invasive biopsy, and wire localization. In addition, each patient was presented at our hospital’s weekly multidisciplinary tumor board conference for review and treatment recommendations.
Surgical technique
A single dose of intravenous antibiotics was given in standard fashion just prior to surgery. Excision of the tumor and surrounding margin was performed, and the surgical area was irrigated with antibiotic solution (Bacitracin). Intra-operative specimen X-ray was obtained, and in most cases, sentinel lymph node biopsy was performed. Next, a sizer set was used to assess the geometry of the excised tumor bed within the surgical cavity in order to assist with selection of the appropriate size tissue marker to be implanted (Fig. 1a-d).
After selection of the appropriate implant, several sutures were placed (typically 4–5 sutures of 3-0 Monocryl) into the margins of the lumpectomy cavity and adjacent mobilized tissue flaps. For deep tumors, where dissection extended down to the pectoralis muscle, securing the implant included sutures into the pectoralis muscle fascia. Caution was taken to avoid deep sutures into the serratus anterior muscle at the lateral aspect of the breast, in order to avoid post-operative pain with movement. The sutures were then secured to the marker thereby approximating the margins of the tumor bed to the implant and securing its position within the tissue (Fig. 2a,b).
Where appropriate, undermining of the skin at the level of the mastectomy plane to create oncoplastic tissue flaps was performed. This allowed enhanced tissue mobility and helped to provide coverage of the implant between the breast and the skin. Multilayer closure was routinely performed. The breasts were immobilized in a compression dressing following surgery, and patients were instructed to wear supportive garments for 7–10 days. Use of the tissue marker implant did not preclude or interfere with performing other procedures typically used during BCS (e.g., sentinel lymph node biopsy, wire localization, reduction mammoplasty, mastopexy).
Radiation therapy
After review of the final pathology report, each case was discussed at the multidisciplinary tumor board, and patients were scheduled for their RT planning CT in the 4–7 week post-op timeframe. Some patients requiring chemotherapy prior to radiation received their initial CT simulations much later (i.e., several months) after surgery. Dose planning was performed using Eclipse™ planning software (Varian Medical, Palo Alto, CA), and in each case, one of two radiation oncologists rated the visibility of the tissue marker on a numeric scale [1–4], as well as the utility of the device for RT planning. The method of RT delivered and the dose regimens (including boost) were recorded and analyzed.
Results
In total, 110 devices were implanted in 108 patients (two patients had bilateral implants placed). Patient demographics are summarized in Table 1, including age, cancer type, nodal status, and re-excision rate.
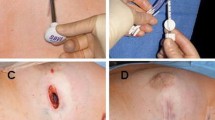
The marker was well tolerated, and in most cases was not palpable. One patient with the implant sutured to the serratus muscle reported discomfort; however, no patients required removal of the device due to palpability or discomfort. Patient photos illustrating typical post-operative outcomes are shown in Fig. 3a, b.
There were no post-operative infections; however, one patient had persistent erythema with fluid at the surgical site. The fluid was drained percutaneously, and cultures were negative. Re-excision was required for close or positive margins in 12.4 % of patients (including two patients requiring mastectomy). Presence of the implant did not impact the ability to perform re-excision, and in some cases where oncoplastic techniques were used, the implant served as a surgical guide to the appropriate area needing re-excision. In most cases, resorption of the implant was noted 16–24 months post-operatively. Prior to resorption, the marker was palpable in some patients. With increased surgical experience in placing the marker, and routine use of the sizer set, palpability of the marker implant was decreased.
Details of the radiation therapy regimens for the patients that have completed RT at the time of this submission are shown in Table 2. One patient was lost to follow-up. Table 2 summarizes the RT results for all patients who have completed RT.
All patients were women, with an average age of 63 years. The average length of time from surgery to planning CT was 37.4 days. In regard to marker visibility during CT simulation, the 3-D marker was rated by both radiation oncologists as “easily visible” in 100 % cases. When scoring the utility of the marker in assisting with dose planning, the marker proved “very useful” or “fairly useful” in 95.7 % of patients. It was rated as “somewhat useful” or “not useful” in 4.3 % of patients. During the course of radiation treatments, the marker assisted with treatment planning, patient positioning and setup using image overlay between fractions from day to day, thereby providing consistency in patient planning and positioning for delivery of RT (Fig. 4a–d).
When summarizing all patients receiving RT, 36.8 % of patients received conventional full-course whole breast irradiation (WBI), 56.6 % received hypo-fractionated WBI, and 6.6 % received accelerated partial breast irradiation (APBI). RT regimens were customized for each patient on a case-by-case basis by the treating radiation oncologist. WBI patients routinely received a boost to the tumor bed, with 61.4 % receiving electrons and 38.6 % receiving photons for the boost dose. When the RT regimen data was analyzed according to three specific timeframes (year one vs. year two vs. year three), an increased use of hypo-fractionation was observed (Fig. 5).
Discussion
In this series of patients, we found use of a novel 3-dimensional tissue marker helped to delineate the surgical margins at the time of partial mastectomy. It proved to have unique features for each of the various clinical specialists involved in the care of the breast cancer patient—the surgeon, the radiation oncologist and the radiologist.
For the surgeon, implantation was intuitive and did not interfere with surgical techniques such as oncoplastic closure, sentinel node biopsy or re-excision for positive margins. It also allowed for approximation of tissue flaps across the center of the device, thereby closing down the cavity and providing a type of “macro-scaffolding” for local breast reconstruction.
Although skin changes, dermal lymphedema, cellulitis and breast deformities were not specifically tracked in this study, we observed that the frequency and severity of undesirable cosmetic changes typically encountered following BCS and radiation appeared to be reduced (Fig. 6). These observations warrant additional study to further document and quantify the extent of potential cosmetic benefits that may be achieved.
In regard to surgical guidance for RT planning, many surgeons use individually placed clips at the edges of the resection cavity. This method is troublesome since the clips mark the extent of the entire resection as opposed to marking the margins of the tumor bed, reflecting the site at greatest risk for recurrence. Additionally, clips can migrate and if distant from the tumor bed, the radiation oncologist may feel compelled to include them in the treatment plan, thereby inadvertently overestimating the treatment volume and including tissue that could potentially have been excluded from the radiation field. These individually placed clips also represent a point source rather than a 3-dimensional region, and since the clips are identical to vascular clips, they can be a source of ambiguity when used as an indicator for delineating the tumor bed.
Other uncertainties in target delineation may be caused by surgical techniques such as oncoplastic cavity closure, tunneling from an incision placed distant from the tumor bed, use of tissue flaps for breast reconstruction or reduction mammoplasty. These techniques make surgical clips and/or the seroma unreliable targets for RT planning since ambiguity and uncertainty arise when trying to precisely visualize the tumor bed on CT [35].
These challenges are well documented in the literature, and prior to the availability of a 3-D marker, there was no reliable surgical solution to assist with RT planning [28, 36–47]. In this study, the marker provided a direct visual means of communication between the surgeon and radiation oncologists for RT planning that decreased dependency on ambiguous planning targets such as the seroma and surrounding tissue changes. This enabled an extended period of time (4–6 week) for surgical healing to occur prior to CT simulation without the risk of losing visualization of the tumor bed. Similarly, the marker maintained a reliable visual target for those patients experiencing a delay in starting treatment—such as those requiring chemotherapy prior to RT. Thus, for the surgeon, the marker provided an efficient, reliable and standardized method for delineating the region of the excised tumor bed as a visual reference.
Our radiation oncology team found that the marker incorporated easily into existing routines for imaging, simulation planning, and delivery methods. The majority of patients in this study were treated with WBI or hypo-fractionated regimens. However, five patients were treated with advanced techniques of RT delivery including: interstitial brachytherapy, 5 field image guided radiation therapy (IGRT) with split arc VMAT, and 3 field external beam radiation therapy. In all but one case, the marker was utilized to determine the size and shape of the treatment field and to assist with patient positioning.
For those patients requiring chemotherapy prior to RT, the presence of the marker consistently maintained the visual cue at the surgical site which proved to be particularly advantageous since these patients had little to no remaining seroma for RT planning. As a result, we noted the dosing and delivery of radiation in these patients was optimally customized and delivered in a more accurate fashion. Overall, the marker proved to be a novel method of marking the tumor excision site and ultimately assisted with daily patient positioning between fractions and treatment planning.
Over 36 months, consistent use of the marker resulted in an increased use of field-in-field RT planning and delivery in our practice. Knowing that the marker had been sutured in place by the surgeon at the tumor excision site, led to an increased confidence when “feathering” or “softening” the radiation dose in non-critical areas. This was done using a shaped treatment beam fashioned with the multileaf collimator on the linear accelerator. This maneuver permitted a reduction in “hot spots” to areas such as the skin, chest wall, etc. As a result, our team became increasingly comfortable with an advanced method of RT known as “hypo-fractionation”. Use of these protocols is becoming increasingly popular, as this method decreases overall treatment time (from 6 to 3–4 weeks) and has a number of additional benefits including significant cost savings [9, 30]. There is a strong interest worldwide to improve targeting in breast RT in order to facilitate an increased use of hypo-fractionation. As seen in Fig. 5, routine use of the surgical marker in our practice led to an associated increase use of hypo-fractionation, which carried with it a 25 % cost reduction per patient treated in this fashion.
Lastly, we found the marker helpful as a guide for long-term follow-up (Fig. 7a, b). It helped guide the radiologist to the area of greatest interest (the tumor excision site) without impeding visualization of the surrounding tissue.
In conclusion, this unique marker provided an effective, straightforward means of visualizing the tumor bed during BCS. It did not interfere with standard surgical techniques, nor were there any device-related complications. The implant provided a consistent and standardized method for RT planning and long-term follow-up and resulted in a significant reduction in planned treatment volumes facilitating use of hypo-fractioned RT. Importantly, when this method of RT was used, a 25 % cost savings per patient was noted. Future studies to quantify cosmesis and comparative radiation treatment volumes when using this device are of significant interest.
References
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Pierce LJ, Griffith KA, Hayman JA, Douglas KR, Lichter AS (2005) Conservative surgery and radiotherapy for stage I/II breast cancer using lung density correction: 10-year and 15-year results. Int J Radiat Oncol Biol Phys 61:1317–1327
Nattinger AB, Hoffmann RG, Kneusel RT, Schapira MM (2000) Relation between appropriateness of primary therapy for early stage breast carcinoma and increased use of breast-conserving surgery. Lancet 356:1148–1153
Bouboul E, Buffat L, Belkacémi Y, Lefranc JP, Uzan S, Lhuillier P, Faivre C et al (1999) Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys 43:25–38
Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola S, Greco M, Luini A, Merson M, Saccozzi R, Rilke F et al (1995) Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 87:19–27
Liljegren G, Holmberg L, Bergh J, Lindgren A, Tabár L, Nordgren H, Adami HO (1999) 10-year results after sector resection with or without postoperative radiotherapy for stage I berast cancer: a randomized trial. J Clin Oncol 17:2326–2333
Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J, Klimberg S et al (2014) Society of Surgical Oncology—American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol 21:704–716
Bekelman JE, Sylwestrzak G, Barron J, Liu J, Epstein AJ, Freedman G, Malin J, Emanuel EJ (2014) Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. JAMA 312(23):2542–2550
Vicini FA (2005) A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer. Radiation Therapy Oncology Group, Philadelphia, PA, protocol #0413
Arthur DW, Vicini FA (2005) Accelerated partial breast irradiation as a part of breast conservation therapy. J Clin Oncol 23:1726–1735
Scanderberg D (2010) Yashar C, White G, Rice R, Pawlicki T. Evaluation of three APBI techniques under NSABP B-39 guidelines. J Appl Clin Med Phys 11(1):274–280
Livi Lorenzo, Meattini I, Marrazzo L, Simontacchi G, Pallotta S, Saieva C, Paiar F et al (2015) Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomized controlled trial. Eur J Cancer 51:451–463
Hepel JT, Leonard KL, Hiatt JR, DiPetrillo TA, Wazer DE (2014) Factors influencing eligibility for breast boost using noninvasive image-guided breast brachytherapy. Brachytherapy 13(6):579–583
Hepel JT, Hiatt JR, Sha S, Leonard KL, Graves TA, Wiggins DL, Mastras D, Pittier A (2014) The rationale, technique, and feasibility of partial breast irradiation using noninvasive image-guided breast brachytherapy. Brachytherapy 13(5):493–501
Murphy JO, Sacchini VS (2013) New innovative techniques in radiotherapy for breast cancer. Minerva Chir 68(2):139–154
Benitez PR, Chen PY, Vicini FA, Wallace M, Kestin L, Edmundson G, Gustafson G, Martinez A (2004) Partial breast irradiation in breast-conserving therapy by way of interstitial brachytherapy. Am J Surg 188:355–364
Xu Q, Chen Y, Grimm J (2012) Dosimetric investigation of accelerated partial breast irradiation (APBI) using CyberKnife. Med Phys 39(11):6621–6628
Chafe S, Moughan J, McCormick B, Wong J, Pass H, Rabinovitch R, Arthur DW et al (2013) Late Toxicity and patient self-assessment of breast appearance/satisfaction on RTOG 0319: A Phase 2 trial of 3-dimensional conformal radiation therapy—accelerated partial breast irradiation following lumpectomy for stages I and II breast cancer. Int J Rad Onc Biol Phys 86(5):854–859
Polgar C, Fodor J, Major T, Takacsi-Nagy Z, Kasler M, Hammer J, Van Limbergen E, Nemeth G (2002) Radiotherapy confined to the tumor bed following breast conserving surgery current status, controversies, and future projects. Strahlenther Onkol 178(11):597–606
Polgar C, Major T, Somogyi A, Takacsi-Nagy Z, Mangel LC, Forrai G, Sulyok Z, Fodor J, Nemeth G (2000) CT-image-bassed conformal brachytherapy of breast cancer. The significance of semi-3-D and 3-D treatment planning. Strahlenther Onkol 176(3):118–124
Benda RK (2003) Yasuda G, Sethi A, Gabram SG, Hinerman RW, Mendenhall NP. Breast boost: are we missing the target? Cancer 97:905–909
Hanbeukers B, van den Ende P, van der Ent F, Houben R, Jager J, Keymeulen K, Murrer L, Sastrowijoto S, van de Vijver K, Boersma L (2009) Customized computed tomography-based boost volumes in breast-conserving therapy: use of three-dimensional histologic information for clinical target volume margins. Int J Radiat Oncol Biol Phys 75(3):757–763
Hepel JT, Evans SB, Hiatt JR, Price LL, DiPetrillo T, Wazer DE, MacAusland SG (2009) Planning the breast boost: comparison of three techniques and evolution of tumor bed during treatment. Int J Radiat Oncol Biol Phys 74(2):458–463
Machtay M, Lanciano R, Hoffman J, Hanks GE (1994) Inaccuracies in using the lumpectomy scar for planning electron boosts in primary breast carcinoma. Int J Radiat Oncol Biol Phys 30(1):43–48
Roth AM, Kauer-Dorner D, Resch A, Schmid A, thill M, Niehoff P, Melchert C, Berger D, Kovacs G (2013) Is oncoplastic surgery a contraindication for accelerated partial breast radiation using the interstitial multicatheter brachytherapy method? Brachytherapy 13(4):394–399
Eblan MJ, Vanderwalde NA, Zeman EM, Jones E (2014) Hypofractionation for breast cancer: lessons learned from our neighbors to the north and across the pond. Oncology 28(6):536–546
Kirby AM, Yarnold JR, Evans PM, Morgan VA, Schmidt MA, Scurr ED, Desouza NM (2009) Tumor bed delineation for partial breast and breast boost radiotherapy planned in the prone position: what does MRI add to X-ray CT localization of titanium clips placed in the excision cavity wall? Int J Radiat Oncol Biol Phys 74(4):1276–1282
van der Laan HP, Dolsma WV, Maduro JH, Korevaar EW, Langendijk JA (2008) Dosimetric consequences of the shift towards computed tomography guided target definition and planning for breast conserving radiotherapy. Radiat Oncol 3:6
Kirby AM, Evans PM, Nerukar AY, Desai SS, Krupa J, Devalia H, della Rovere GQ, Harris EJ, Kyriakidou J, Yarnold JR (2010) How does knowledge of three-dimensional excision margins following breast conservation surgery impact upon clinical target volume definition for partial breast radiotherapy? Radiother Oncol 94(3):292–299
Peterson D, Truong PT, Parpia S, Olivottto IA, Berrang T, Kim DH, Kong I, Germain I et al (2015) Predictors of adverse cosmetic outcome in the RAPID trial: an exploratory analysis. Int J of Rad Onc Biol Phys 91(5):968–976
Smith LA, Kuske RR, Cross MJ (2014) Improved targeting of the lumpectomy cavity using a spiral 3-dimensional marker. Poster presentation; Am Soc Ther Rad Onc (ASTRO), San Francisco, October, (2014)
Harman J, Govender S, Benjamin B, Simpson J (2014) Poster presentation; An improved method for marking the surgical cavity during partial mastectomy. Royal Aust New Zealand Conf Clin Radiol (RANZCR), Auckland, October, (2014)
Kaufman CS, Hall W, Hill L, Caro R, Nix S, Evans E, Zacharias K et al (2015) Poster presentation; Initial experience with a novel 3-dimensional bioabsorbable lumpectomy marker. Am Soc of Breast Surgeons, Orlando, April
Eaton BR, Losken A, Okwan-Duodu D, Schuster DM, Switchenko JM, Mister D et al (2014) Local recurrence patterns in breast cancer patients treated with Oncoplastic reduction mammoplasty and radiotherapy. Ann Surg Onc 21:93–99
Landis DM, Luo W, Song J, Bellon JR, Punglia RS, Wong JS, Killoran JH, Gelman R, Harris JR (2007) Variability among breast radiation oncologists in delineation of the postsurgical lumpectomy cavity. Int J Radiat Oncol Biol Phys 67:1299–1308
Rabinovitch R, Finlayson C, Pan Z, Lewin J, Humphries S, Biffi W, Francoise R (2000) Radiographic evaluation of surgical clips is better than ultrasound for defining the lumpectomy cavity in breast boost treatment planning: a prospective clinical study. Int J Radiat Oncol Biol Phys 47(2):313–317
Harrington KJ, Harrison M, Bayle P, Evans K, Dunn PA, Lambert HE, Saidan Z, Lynn J, Stewart JS (1996) Surgical clips in planning the electron boost in breast cancer: a qualitative and quantitative evaluation. Int J Radiat Oncol Biol Phys 34(3):579–584
Penninkhof J, Quint S, Boer HD, Mens JW, Heijmen B, Dirkx M (2009) Surgical clips for position verification and correction of non-rigid breast tissue in simultaneously integrated boost (SIB) treatments. Radiother Oncol 90(1):110–115
Coles CE, Wilson CB, Cumming J, Benson JR, Forouhi P, Wilkinson JS, Jena R, Wishart GC (2009) Titanium clip placement to allow accurate tumour bed localization following breast conserving surgery: audit on behalf on the IMPORT Trial Management Group. Eur J Surg Oncol 35(6):578–582
Pirlamarla A, Ferro A, Yue NJ, Haffty BG, Goyal S (2014) Optimization of surgical clip placement for breast-conservation therapy. Pract Radiat Oncol 4:153–159
Coles CE, Harris EJ, Donovan EM, Bliss P, Evans PM, Fairfoul J, Mackenzie C, Rawlings C et al (2011) Evaluation of implanted gold seeds for breast radiotherapy planning and on treatment verification: a feasibility study on behalf of the IMPORT trialists. Radiother Oncol 100:276–281
Shaikh T, Chen T, Khan A, Yue NJ, Kearney T, Cohler A, Haffty BG, Goyal S (2010) Improvement in interobserver accuracy in delineation of the lumpectomy cavity using fiducial markers. Int J Radiat Oncol Biol Phys 78:1127–1134
Kirby AM, Evans PM, Nerurkar AY, Desai SS, Krupa J, Devalia H, della Rovere GQ et al (2010) How does knowledge of three-dimensional excision margins following breast conservation surgery impact upon clinical target volume definition for partial-breast radiotherapy? Radiother Oncol 94:292–299
Kirby AM, Coles CE, Yarnold JR (2010) Target volume definition for external beam partial breast radiotherapy: clinical, pathological and technical studies informing current approaches. Radiother Oncol 94:255–263
Njeh CF (2008) Tumor delineation: the weakest link in the search for accuracy in radiotherapy. J Med Phys 33:136–140
Giezen M, Kouwenhoven E, Scholten AN, Coerkamp EG, Heijenbrok M, Jansen WP, Mast ME et al (2010) MRI versus CT-based volume delineation of lumpectomy cavity in supine position in breast-conserving therapy: an exploratory study. Int J Radiat Oncol Biol Phys 82:1332–1340
Disclosures
Dr. Lebovic is a consultant for Focal Therapeutics, Inc., Aliso Viejo, California.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Cross, M.J., Lebovic, G.S., Ross, J. et al. Impact of a Novel Bioabsorbable Implant on Radiation Treatment Planning for Breast Cancer. World J Surg 41, 464–471 (2017). https://doi.org/10.1007/s00268-016-3711-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3711-y