Abstract
Background
The BRAF V600E mutation is a recognised molecular marker in papillary thyroid cancer (PTC), reported incidence from 30 to 80 %. BRAFV600E aberrantly activates the MAPK pathway, a central regulator of cell growth and proliferation. Previous studies have reported conflicting data regarding the impact of BRAFV600E on clinicopathological features of PTC. The study aims to determine whether BRAFV600E is useful as a prognostic biomarker in PTC.
Methods
A cohort study of patients undergoing surgery for PTC was undertaken. The primary outcome measure was disease-free survival. Secondary outcome measures were tumour size, nodal positivity and radioactive iodine ablation rate. All cases were re-examined to confirm PTC. Immunohistochemistry for BRAFV600E was performed on tissue microarrays. A single endocrine pathologist, blinded to clinicopathological data, interpreted staining.
Results
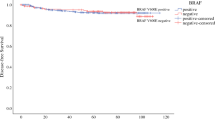
496 patients with PTC were included, and 309 (62 %) were BRAFV600E positive. Tumour size was similar for BRAFV600E-positive and -negative tumours (21.3 vs. 23.2 mm, p = 0.23). BRAFV600E-positive patients were significantly older at first operation (mean age 45 versus 49 years, p = 0.003). BRAFV600E-positive PTCs had a higher rate of disease recurrence (12.9 vs. 5.6 %, p = 0.004), lymph node metastasis (44 vs. 29.4 %, p = 0.004) and extra-thyroidal extension (44 vs. 22 %, p < 0.001). Five-year disease-free survival was 89.6 % for BRAFV600E positive and 96.3 % for negative tumours, p < 0.001. There was no difference between groups for vascular invasion or multifocality. The mean follow-up was 57 months for both groups.
Conclusion
BRAFV600E in PTC predicts an increased risk of lymph node metastasis, extra-thyroidal extension and reduced disease-free survival. It is an additional useful prognostic biomarker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the commonest endocrine malignancy, comprising 1 % of all cancers. 80–85 % of thyroid cancer cases are papillary thyroid cancer (PTC) [1]. Generally, PTC has a good long-term prognosis with a 10-year survival rate over 90 % [1]. However, a subgroup of patients develops more aggressive disease. These patients experience disease recurrence with associated morbidity and mortality, with an estimated 35,000 people dying annually from thyroid cancer worldwide [2]. Surgical options are more restricted and difficult when disease recurs, particularly when tumours become refractory to radioactive iodine.
Clinical guidelines have been developed to stratify patients into different risk groups. This allows clinicians to tailor more aggressive treatment and closer follow-up to patients considered high risk. Currently, these guidelines are based on clinicopathological risk factors including patient age, gender, co-morbidities, tumour size, histological type, lymph node involvement and extra-thyroidal spread [3–6]. However, risk stratification is often inaccurate with 15 % of tumour recurrence and 10 % of thyroid cancer mortality accounted for by patients deemed “low risk” by such criteria [2, 7, 8]. Therefore, clinicians have endeavoured to develop better prognostic algorithms and recently molecular tumour markers have gained widespread interest.
Genetic alterations frequently occur in PTC, the most common within the gene encoding BRAF kinase, c.1799T > A, p.Val600Glu (BRAF V600E), with a reported prevalence ranging from 29 to 83 % [6, 9]. BRAF activates the mitogen-activated protein kinase (MAPK) intracellular signal transduction pathway. The MAPK pathway has a central role in regulation of gene expression, cell growth, proliferation and survival [10, 11]. BRAF V600E accounts for over 95 % of BRAF mutations and is oncogenic by constitutive MAPK activation [9, 12]. BRAF V600E occurs almost exclusively in PTC and PTC-derived anaplastic carcinoma [6]. Consequently, BRAF has been investigated for its tumorigenic and prognostic value in clinical practice, although initial clinical results were mixed. Whereas many studies demonstrated BRAF V600E to be associated with parameters of tumour aggressiveness [6, 13–16], others found no association [17–19].
This controversy has mainly been resolved by larger multi-centre studies and meta-analyses. These have confirmed association between BRAF V600E and advanced tumour characteristics, especially extra-thyroidal extension, lymph node involvement and stage III and IV disease [2, 10, 14–16, 20]. More recently, studies have investigated the association of BRAF V600E with recurrence, demonstrating BRAF V600E-positive patients have an increased chance of recurrence. However, these reports were mostly single-institution studies with small patient series [13, 16, 21–24]. A recent international multi-centre study demonstrated that BRAFV600E-positive patients have a higher chance of recurrence, even in conventionally low-risk stage 1 and 2 and micro-PTC [24]. The aim of this study was to evaluate BRAF mutation as a prognostic biomarker in PTC.
Materials and methods
This cohort study involved 525 consecutive patients with PTC who underwent surgery from 1990 to 2012 at the University of Sydney Endocrine Surgery Unit. Cases were identified from a prospectively maintained thyroid surgery database, after approval from the local institutional human research ethics committee. Paraffin-embedded tissue was available only from the Department of Anatomical Pathology at the Royal North Shore Hospital, with acquisition regulated by the New South Wales Human Tissue Act 1983. Hence, the number of patients in this study does not represent the full clinical workload of the unit during this time.
The primary outcome measure was disease-free survival, defined as the absence of structural PTC recurrence during follow-up. Structural recurrence was defined as disease that was visible on cross-sectional imaging (ultrasound, CT, MRI or PET/CT) and confirmed to be papillary thyroid carcinoma on cytology or histopathology, independent of serum thyroglobulin levels. This definition therefore excludes patients with slight elevations in serum thyroglobulin and no evidence of disease on imaging. Secondary outcome measures were tumour size, cervical lymph node positivity and rate of radioactive iodine ablation.
All cases were reviewed centrally to confirm diagnosis of PTC. Tissue microarrays (TMAs) were constructed from formalin-fixed paraffin-embedded (FFPE) tissue, containing 2 × 1 mm cores from each tumour. Sufficient material was present for immunohistochemistry (IHC) in the TMA sections of 496 patients. BRAF V600E mutation-specific IHC was performed using a commercially available mouse monoclonal antibody (clone VE1, Spring Bioscience, Pleasanton, CA) using the methods we previously described [25]. Briefly, VE1 IHC was performed using the Leica Bond-III autostainer (Leica Microsystems, Mount Waverley, VIC, Australia) used according to the manufacturer’s protocol with alkaline antigen retrieval (solution ER2, VBS part no: AR 9640, Leica Microsystems) with the primary antibody used at a dilution of 1 in 80.
BRAFV600E staining was interpreted as positive if there was any positive cytoplasmic staining in neoplastic cells. Staining in colloid and non-neoplastic cells was disregarded. Staining was interpreted by a single experienced endocrine pathologist (AG), blinded to all clinicopathological data (including results of BRAF testing if performed using DNA-based methods). 101 cases from this cohort had previously undergone IHC for BRAFV600E on whole sections plus molecular testing by Sanger Sequencing and restriction fragment length polymorphism (RFLP) in a previous study, in which IHC appeared superior to Sanger sequencing and RFLP [25]. As a quality assurance measure, the results of cases scored blinded in TMA format were compared to the results of IHC previously performed on whole sections and compared to molecular testing in the Bullock et al. study [25].
Statistical analysis was undertaken using STATA software. Continuous variables were assessed by the independent samples t test or the Mann–Whitney U test. Categorical variables were compared using the Fisher exact test or Pearson’s Chi-squared test where appropriate. Kaplan–Meier survival curves, log-rank tests and Cox proportional regression analysis, at time of last follow-up, were used to compare recurrence by BRAF V600E mutation status and other factors. A p value of <0.05 was considered significant.
Results
Of 525 patients identified in the thyroid cancer database, 29 were excluded due to an inadequate amount of tissue to assess BRAF status by IHC. There were 496 remaining patients: 309 (62 %) were BRAFV600E positive and 187 (38 %) were negative. There was complete concordance between the IHC results interpreted on the TMA when compared to that previously performed on whole sections [25] in the cases which appeared in both cohorts (n = 101).
Patient demographics at baseline are shown in Table 1. Patients with BRAFV600E-positive PTC were significantly older at the primary operation (p = 0.003). Clinicopathological characteristics at baseline are shown in Table 2.
BRAFV600E-positive PTCs were associated with a significantly higher rate of extra-thyroidal extension—44 % compared to 22 % in BRAFV600E-negative patients (p < 0.001). More BRAFV600E-positive patients underwent a central lymph node dissection (CND) at their initial operation (p < 0.001). BRAFV600E-positive patients who underwent a CND had more lymph nodes removed and a higher number of positive lymph nodes, 44 % compared to 29.4 % (p = 0.004). Routine CND is standard procedure in this unit for patients with PTC over 1 cm in maximum diameter. This has been incorporated into our protocols since 2003, and BRAF status was not known at the original surgery.
Tumour size was similar for BRAFV600E-positive and -negative tumours (21.3 vs. 23.2 mm, p = 023). There was no significant association between BRAFV600E positivity and vascular invasion or multifocal disease.
Disease outcomes are shown in Table 3. Follow-up was equal for both groups, with a mean of 57 months from the primary operation, range 2–517 months for BRAFV600E negative and 2–528 months for positive patients. There was no difference between groups in the proportion of patients (87.5 % both groups) who received radioactive ablation (RAI) treatment. There was no difference in the number or overall doses of RAI received between groups.
The number of patients diagnosed with distant metastases was extremely low at diagnosis, only 0.02 % in each group. Although the time to structural recurrence was similar between the groups, the percentage recurrence during follow-up was significantly higher in patients with BRAFV600E-positive PTC; overall, 12.9 % of BRAFV600E-positive patients had structural recurrence compared to only 5.3 % of BRAFV600E negative. Disease-free survival at five years was adversely affected by BRAFV600E status; 96.3 % of BRAFV600E-negative patients were disease free, compared to 89.6 % of BRAFV600E positive. Locoregional structural recurrence was detected in patients on follow-up by either palpation and/or imaging. This includes ultrasound, CT, MRI or PET/CT and is independent of thyroglobulin levels. In multivariate analysis using Cox proportional hazards model, gender, tumour stage and BRAFV600E status were significantly associated with structural recurrence. The risk of local recurrence is increased by 2-fold on multivariate analysis for BRAF V600E-positive patients, in contrast to BRAFV600E negative (Table 4).
Figure 1 demonstrates the significant reduction in disease-free survival with T stage, according to the tumour-node-metastasis (TNM) cancer staging system, developed by the American Joint Commission on Cancer. BRAF V600E-positive patients were shown to have a significant reduction in disease-free survival (Fig. 2), compared to BRAF V600E negative. When outcome was analysed in subgroups, according to T stage, BRAF V600E positivity was established as an independent indicator for poor prognosis in T2, T3 and T4 tumours (Figs. 3, 4).
Discussion
This study documents cancer-related outcomes in a large cohort of patients with PTC and confirms a strong association between BRAF positivity and reduced disease-free survival. We believe these data support the incorporation of BRAF immunohistochemical staining into routine histopathological reporting of well-differentiated thyroid cancer, especially in units where routine central node dissection does not occur.
The incidence of thyroid cancer has been steadily rising worldwide. SEERS (Surveillance, Epidemiology and End Results) data from the USA have demonstrated that over the past ten years, the rates of new thyroid cancer cases have risen on average by 5.5 % each year, with an associated increased mortality by 0.8 % per annum. This rise is mirrored in other countries including the United Kingdom (Office for National Statistics) and Australia (Cancer Australia Statistics).
The rise in thyroid cancer is considered both an apparent and true increase. The escalating use of sensitive radiological investigations has identified a number of small, asymptomatic cancers, which may have remained clinically undetectable. However, the number of larger and more advanced thyroid cancers has also increased, notably only the PTC subtype [26, 27]. In contrast to most other cancers, including breast, colorectal, prostate and lung, where mortality has decreased over the past two decades, mortality from thyroid cancer has slightly risen [26, 28].
It is therefore increasingly important to differentiate which patients with PTC are higher risk for more aggressive disease and a greater chance of recurrence, compared to the majority with more benign disease. Molecular markers are being investigated for use as potential prognostic biomarkers, which could help in planning personalised treatment [29].
The most common genetic mutations associated with PTC are RET/PTC rearrangement and activating mutations in BRAF. RET/PTC is correlated with radiation exposure and childhood PTC. In contrast, BRAFV600E is usually seen in adults with a more aggressive phenotype [29]. BRAFV600E has a higher incidence in conventional PTC and tall cell variants, but is less common in follicular variant PTC [30].
This study assesses the impact of BRAFV600E in a large consecutive cohort of patients treated for thyroid cancer at a single-tertiary Australian institution. The proportion of BRAFV600E-positive PTCs in our cohort is consistent with previous reports [14, 30].
Along with previous studies, we have demonstrated that BRAFV600E correlates with more aggressive clinicopathological features, including extra-thyroidal extension and cervical lymph node involvement. BRAFV600E-positive patients had a higher rate of initial lymph node dissection, with a significantly greater percentage of metastatic lymph nodes. The association between BRAFV600E-positive PTC and cervical lymph node metastases is clinically relevant as PTC typically recurs in the cervical lymph nodes [31]. Along with other studies, we did not demonstrate that positive BRAF V600E status had an impact on distant metastases or overall survival [32]. However, our most significant finding was that BRAFV600E is associated with reduced disease-free survival, independent of age, sex and tumour stage.
A recently published retrospective single-institution cohort study, also with just over 500 patients, did not demonstrate that BRAFV600E had an adverse effect on recurrence. However, in keeping with our findings, BRAFV600E strongly correlated with cervical lymph node metastases, 75 % with lymph node involvement compared 25 % of BRAFV600E-negative patients. The strongest predictor of recurrence in that series was cervical lymph node metastases [33].
Total thyroidectomy with prophylactic CND is routinely performed in our unit for patients with a pre-operative diagnosis of PTC. However, the role of prophylactic CND remains controversial. The latest British guidelines for the management of differentiated thyroid cancer do not advocate prophylactic CND for patients with small tumours and no high-risk features, whilst in high-risk patients it is left to the discretion of the individual surgeon [34]. The American Thyroid Association has a similar recommendation, with prophylactic CND only recommended in patients with advanced primary tumours [3]. On the basis of our results, we would advocate that BRAFV600E-positive patients should undergo a prophylactic CND, due to a higher likelihood of lymph node metastases and reduced disease-free survival.
Radioactive iodine ablation (RAI) is current accepted practice after total thyroidectomy in the majority of patients with PTC. Several recent studies, however, suggest that RAI can be dose-reduced (or even avoided) in patients with low-risk disease. A recent multi-centre trial has demonstrated that in low-risk thyroid cancer, low-dose RAI is as effective as high doses, with less adverse effects [35].
Current clinical guidelines that stratify patients as low or high risk are based on clinicopathological features, not considering molecular markers. Whether BRAF status should be included in treatment algorithms requires further study to demonstrate that RAI specifically reduces recurrence risk in BRAF-positive cases.
Targeted molecular therapies are being considered in patients with recurrent or resistant PTC. Sorafenib is one example, with activity against multiple tyrosine kinases, including RAF, c-KIT, platelet-derived growth factor (PDGF) and vascular endothelial growth factor receptor (VEGFR) 2 and 3 [36]. A recent randomised double-blinded phase III trial assessed its efficacy in patients with RAI-refractory locally advanced or metastatic differentiated thyroid cancer. Whereas sorafenib improved progression-free survival, this was independent of BRAF mutation status [37], possibly due to relatively weak action against BRAF kinase [36].
Vemurafenib is a potent kinase inhibitor of BRAFV600E currently licensed for use in unresectable or metastatic melanoma. The first-phase I trial was in patients with metastatic melanoma and the BRAF V600E mutation. A clinical response was demonstrated in BRAFV600E-positive patients only. A further phase I trial with vemurafenib was carried out in three BRAFV600E-positive patients with metastatic PTC. A partial response was seen in one patient and prolonged stabilisation of disease in the other two patients. An international multi-centre phase II trial is now underway [38]. Initial results with 51 BRAFV600E-positive patients are encouraging, with a median progression-free survival of 15.6 months in patients having not received previous TKI therapy.
Conclusion
BRAFV600E positivity in our cohort of patients was a predictor of worse disease-related outcomes in PTC, independent of traditional risk factors. Our data demonstrate that BRAFV600E is associated with more aggressive clinicopathological features, a higher risk of structural recurrence and reduced disease-free survival.
Clinical guidelines categorising patients into low- or high-risk groups do not yet consider BRAFV600E status. However, due to the ongoing controversy around the extent of initial surgery in PTC, RAI dosages and new emerging treatments targeted at the BRAF V600E mutation, BRAF V600E mutation testing by immunohistochemistry should be considered in the routine histological evaluation of papillary thyroid carcinoma.
References
Schlumberger MJ (1998) Papillary and follicular thyroid carcinoma. N Engl J Med 338(5):297–306
Tang KT, Lee CH (2010) BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc 73(3):113–128
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ et al (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19(11):1167–1214
Dean DS, Hay ID (2000) Prognostic indicators in differentiated thyroid carcinoma. Cancer Control 7(3):229–239
Gilliland FD, Hunt WC, Morris DM, Key CR (1997) Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer 79(3):564–573
Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ et al (2005) BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90(12):6373–6379
Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T et al (1999) Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab 84(11):4043–4049
Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP (1997) Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab 82(11):3553–3562
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S et al (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892):949–954
Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M et al (2008) BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer 15(1):191–205
Mercer KE, Pritchard CA (2003) Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta 1653(1):25–40
Garnett MJ, Marais R (2004) Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6(4):313–319
Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ et al (2012) The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118(7):1764–1773
Lee JH, Lee ES, Kim YS (2007) Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 110(1):38–46
Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI et al (2003) Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab 88(9):4393–4397
Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F et al (2003) BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88(11):5399–5404
Fugazzola L, Mannavola D, Cirello V, Vannucchi G, Muzza M, Vicentini L et al (2004) BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf) 61(2):239–243
Liu RT, Chen YJ, Chou FF, Li CL, Wu WL, Tsai PC et al (2005) No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin Endocrinol (Oxf) 63(4):461–466
Puxeddu E, Moretti S, Elisei R, Romei C, Pascucci R, Martinelli M et al (2004) BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab 89(5):2414–2420
Kim KH, Kang DW, Kim SH, Seong IO, Kang DY (2004) Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J 45(5):818–821
Elisei R, Viola D, Torregrossa L, Giannini R, Romei C, Ugolini C et al (2012) The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab 97(12):4390–4398
Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G et al (2006) The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 65(3):364–368
Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P (2006) The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer 13(1):257–269
Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D et al (2015) Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 33(1):42–50
Bullock M, O’Neill C, Chou A, Clarkson A, Dodds T, Toon C et al (2012) Utilization of a MAB for BRAF(V600E) detection in papillary thyroid carcinoma. Endocr Relat Cancer 19(6):779–784
Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol: 965212
Vigneri R, Malandrino P, Vigneri P (2015) The changing epidemiology of thyroid cancer: why is incidence increasing? Curr Opin Oncol 27(1):1–7
Chen AY, Jemal A, Ward EM (2009) Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer 115(16):3801–3807
Ringel MD (2009) Molecular markers of aggressiveness of thyroid cancer. Curr Opin Endocrinol Diabetes Obes 16(5):361–366
Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh QY et al (2007) The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246(3):466–470
Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A (2012) Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5768 patients with average 10-year follow-up. World J Surg 36(6):1274–1278
Niederer-Wust SM, Jochum W, Forbs D, Brandle M, Bilz S, Clerici T et al (2015) Impact of clinical risk scores and BRAF V600E mutation status on outcome in papillary thyroid cancer. Surgery 157(1):119–125
Henke LE, Pfeifer JD, Ma C, Perkins SM, DeWees T, El-Mofty S, et al (2015) BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma. Cancer Med
Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G et al (2014) Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 81(Suppl 1):1–122
Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L et al (2012) Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med 366(18):1674–1685
Melck AL, Yip L, Carty SE (2010) The utility of BRAF testing in the management of papillary thyroid cancer. Oncologist 15(12):1285–1293
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L et al (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384(9940):319–328
Kim KB, Cabanillas ME, Lazar AJ, Williams MD, Sanders DL, Ilagan JL et al (2013) Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid 23(10):1277–1283
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fraser, S., Go, C., Aniss, A. et al. BRAFV600E Mutation is Associated with Decreased Disease-Free Survival in Papillary Thyroid Cancer. World J Surg 40, 1618–1624 (2016). https://doi.org/10.1007/s00268-016-3534-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3534-x