Abstract
Background
Significance of splenic hilar node dissection with splenectomy is now denied for advanced gastric cancer of upper one-third of the stomach without invasion to the greater curvature by the Japan Clinical Oncology Group 0110, a pivotal randomized study from Japan. However, a question remains for tumors which involve the greater curvature, as this study excluded such tumors.
Methods
We retrospectively analyzed 421 consecutive patients with gastric cancer who underwent curative total gastrectomy with splenectomy from 1992 to 2009. The survival curves, state of lymph node (LN) metastasis, and index of the estimated benefit from LN dissection of each station were evaluated according to the tumor location.
Results
The incidence of No. 10 metastasis was 9.3 % (39/421), with 15.9 % in patients with tumors involving the greater curvature (Gre group, n = 132) and 6.2 % in those without (non-Gre group, n = 289) (P = 0.032). The 5-year overall survival (OS) of patients with and without No. 10 metastasis was 35.4 and 43.1 % (P = 0.135) in the Gre group and 32.8 and 66.5 % (P = 0.0006) in the non-Gre group, respectively. The index of No. 10 LN dissection was 5.6 and 2.0 in the Gre and non-Gre groups, respectively. In the Gre group, the index was relatively higher in patients aged < 65 years, within pT3, and with Borrmann type 4 tumors.
Conclusions
Splenectomy may have a survival benefit when a tumor shows involvement with the greater curvature, especially in relatively young patients and those without serosal exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, total gastrectomy with simultaneous splenectomy has been performed for complete removal of the splenic hilar lymph nodes (LNs), which constitute the No. 10 station according to the classification of the Japanese Gastric Cancer Association (JGCA) [1], in the treatment of advanced proximal gastric cancer. This procedure, termed D2 total gastrectomy, has been also recommended in the 2010 guideline of the JGCA [2]. The incidence of No. 10 metastasis in patients with advanced proximal gastric cancer is reportedly 10–26 % [3, 4]; however, some publications have noted its association with high morbidity and mortality, and no significant survival benefit [5–8]. Especially in Western countries, splenectomy for D2 dissection is not commonly performed based on the results of two large randomized trials conducted in European countries [9, 10].
However, some researchers have insisted on the survival benefit of splenectomy. Kosuga et al. performed a retrospective analysis and found that patients with tumors localized on the greater curvature may obtain a survival benefit by undergoing splenectomy [11]. It seems probable that a subpopulation of patients benefits from splenectomy; if so, identification of the most suitable candidates is important. As suggested by other authors [4, 12], tumor location is likely to be a pivotal clinical factor in this issue. The Japan Clinical Oncology Group (JCOG) conducted a phase-III randomized clinical trial (JCOG 0110) comparing splenectomy versus non-splenectomy in 505 patients with tumors not involving the greater curvature [13]. The results of the JCOG 0110 were recently presented at a congress and showed the non-inferiority of spleen preservation to splenectomy in terms of overall survival (OS) [14]. Regardless of the results of the JCOG 0110, clinical questions concerning tumors involving the greater curvature remain.
In our institution, total gastrectomy with splenectomy has long been a standard therapy for proximal advanced gastric cancer. The current retrospective analysis was conducted to assess the survival benefit of this surgery using our clinical database, including long-term outcomes. A subgroup analysis according to tumor location was also performed to identify the most appropriate candidates for this surgery.
Materials and methods
Patients
We retrospectively reviewed the clinical records of 421 patients with proximal gastric cancer who underwent R0 total gastrectomy with simultaneous splenectomy (D2) from 1992 to 2009 at the National Cancer Center Hospital East in Japan. Patients with remnant gastric cancer, double cancer, and intraoperatively confirmed metastasis to para-aortic LNs were excluded.
Surgical procedure and follow-up
Standard Japanese D2 total gastrectomy with splenectomy was performed by surgeons experienced in laparotomy. The pancreas was basically preserved, leaving the splenic artery and vein as distal as possible to maintain vessel communication with the pancreas tail. Simultaneous pancreas resection was undertaken only when direct invasion from the primary tumor or metastatic LNs was recognized. Since 2007, S-1 has been the standard postoperative chemotherapy regimen based on the results of the ACTS-GC trial in Japan [15]; therefore, postoperative adjuvant chemotherapy was carried out when the final tumor stage was consistent with the ACTS-GC criteria. Outpatient follow-up involved physical examination, blood tests including tumor marker evaluation, and chest/abdominal computed tomography scans every 3 months for the first 2 years and then every 6 months until at least 5 years postoperatively.
Clinical parameters
We reviewed the following clinical and pathological factors: sex, age, maximum tumor size, macroscopic type according to the Borrmann classification, tumor location, morbidity and mortality rates, length of postoperative hospital stay, histological type, pathological T and N factors, and stage. Definition of tumor’s cross-sectional circumferential location followed JGCA classification [1], in which the stomach wall is divided into four equal parts. Pathological and surgical reports were reviewed to decide circumferential location of the tumors. JGCA classification of gastric cancer was also used to describe tumor progression and histological grading. Histopathological diagnosis was performed by experienced pathologists. Postoperative complications were classified using the Clavien–Dindo grading system [16], and grade III or higher represented morbidity.
Incidence of LN metastasis, long-term outcomes, and index of estimated benefit of LN dissection
The incidence of LN metastasis was determined using the final pathological reports. The number of regional LNs was categorized according to the JGCA classification [1]. We evaluated the OS curve, which was further stratified by several clinical factors. Finally, we evaluated the index of the estimated benefit of LN dissection of each station, which was calculated by multiplying the incidence of metastasis to a station by the 5-year survival rate of patients with metastasis to this station [17].
Statistics
All statistical analyses were performed using JMP version 11 (SAS Institute, Cary, NC). The Chi-squared test and Student’s t test were used for statistical analysis. Survival curves were constructed by the Kaplan–Meier method, and the log-rank test was used to assess survival differences. All P values of < 0.05 were considered statistically significant.
Results
Clinicopathological characteristics
The patients’ clinicopathological characteristics are summarized in Table 1. The patients were divided into two groups: those with gastric cancer involving a cross-sectional quarter part of the greater curvature site (Gre group, n = 132) and those with gastric cancer not involving Gre (non-Gre group, n = 289). The Gre group included larger tumors, deeper invasion to the wall, and more LN metastases, resulting in more advanced stages. The incidence of type 4 tumors was much higher in the Gre than non-Gre group (42.4 vs. 4.5 %, respectively; P < 0.001), and the incidence of histologically undifferentiated tumors was also higher (70.8 vs. 46.4 %, respectively; P < 0.001). Pancreaticosplenectomy was performed in 14 patients in the Gre group (10.6 %) and 26 in the non-Gre group (9.0 %) with no significant difference (P = 0.59). The incidence of adjuvant chemotherapy was not different between the two groups.
Morbidity and mortality
Among all patients (n = 421), the incidence of pancreatic fistulas or related peritoneal abscesses was 19.7 %, and that of anastomotic leakage was 5.9 %. Grade III or higher postoperative complications occurred in 111 (26.4 %) patients, 16 (14.4 %) of whom underwent simultaneous pancreatic resection. Two (0.47 %) patients died of postoperative intra-abdominal bleeding and severe pneumonia, respectively. The median length of the postoperative hospital stay was 21 days (range 7–181 days).
LN metastasis
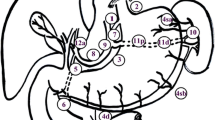
The overall incidence of No. 10 metastasis was 9.3 % (39/421); 15.9 % (21/132) in the Gre group and 6.2 % (18/289) in the non-Gre group, with a significant difference [P = 0.032, 95 % confidence interval (95 % CI) 1.46–5.55, OR = 2.85]. The incidence of metastasis to regional LN stations in both groups is shown in Fig. 1. Metastasis was generally more often recognized in the lesser curvature (Nos. 1, 3, and 7), but in the Gre group, the rate of metastasis to No. 10 was similar to that of other important stations (Nos. 2, 4s, 6, and 7).
Incidence of lymph node (LN) metastasis and index in the Gre and non-Gre groups. a In the Gre group, the incidence and index of station No. 10 was similar to those of Nos. 2, 4s, 6, and 7. b In the non-Gre group, the incidence and index of station No. 10 were relatively lower than those of other LN stations. 1 Right paracardial LNs, 2 left paracardial LNs, 3 LNs along the lesser curvature, 4s LNs along the short gastric or right gastroepiploic vessels, 4d LNs along the right gastroepiploic vessels, 5 Suprapyloric LNs, 6 Infrapyloric LNs, 7 LNs along the left gastric artery, 8a LNs along the common hepatic artery, 9 LNs around the celiac artery, 10 LNs at the splenic hilum, 11 LNs along the splenic artery, 12a LNs in the hepatoduodenal ligament (along the hepatic artery)
Survival outcomes and index of estimated benefit of LN dissection
The 5-year OS in the Gre group was 41.9 %, and that in the non-Gre group was 64.5 %. There was a significant difference in the 5-year OS (P < 0.001, 95 % CI 1.48–2.63, HR = 1.98) (Fig. 2). Survival rates were stratified according to pathological stage; the 5-year OS rates in the Gre and non-Gre groups were 100.0 and 71.8 % for stage IB, 50.9 and 70.0 % for stage IIA, 52.3 and 76.7 % for stage IIB, 50.0 and 68.3 % for stage IIIA, 26.6 and 46.9 % for stage IIIB, and 27.1 and 29.4 % for stage IIIC, respectively. Overall, the 5-year OS rates with and without No. 10 metastasis were 34.2 and 59.6 %, respectively (P = 0.0001, 95 % CI 1.36–3.17, HR = 2.12) (Fig. 3a). In the non-Gre group, the 5-year OS rates with and without No. 10 metastasis were 32.8 and 66.5 %, respectively, with a significant difference (P = 0.0006, 95 % CI 1.43–4.82, HR = 2.75) (Fig. 3b). In the Gre group, however, these rates were 35.4 and 43.1 %, respectively, with no significant difference (P = 0.135) (Fig. 3b). The calculated index of the estimated benefit of LN dissection of each station is shown in Fig. 1. In the Gre group, the index of No. 10 was 5.6, which was lower than that of Nos. 1, 3, and 4d, but similar to that of Nos. 2, 4s, 7, and 11. In the non-Gre group, the index of No. 10 was 2.0, which was relatively lower than that of other LN stations.
a Survival curve for all patients with and without No. 10 metastasis. Patients with No. 10 metastasis had lower survival rates (P = 0.0001, HR = 2.12). b Survival curve for patients in the non-Gre group with and without No. 10 metastasis. There was a significant difference between the two groups (P = 0.0006, HR = 2.75). c Survival curve for patients in the Gre group with and without No. 10 metastasis. There was no significant difference (P = 0.135)
Index according to clinical factors in patients with No. 10 metastasis in Gre group
Because the index was relatively high in the Gre group (5.6), it was further analyzed according to various clinicopathological factors. The results are shown in Table 2. The indices of patients aged < 65 years (8.2), with Borrmann type 4 tumors (7.1), and with stage pT2 to pT3 disease (10.0) were relatively high. Regarding Borrmann type 4, No. 10 metastasis was recorded in eight patients (14.2 %), and the depth of tumor invasion in those patients were pT2 in 2, pT3 in 3, and pT4 in 3.
Discussion
The survival benefit of splenectomy has long been debated, and both positive and negative opinions have been published. A Korean group reported that splenectomy had no survival benefit when metastatic LNs existed at the splenic hilum or along the splenic artery [18]. A Chinese group reported that No. 10 metastasis is a predictor of a worse prognosis and that there was no significant difference in survival between R0 resection and R1–2 resection when No. 10 metastasis was present [19]. They also assumed that No. 10 LN metastasis is basically associated with more extensive spread of cancer, including to the para-aortic LNs, so performance of surgical resection with an expectation of long survival is questionable. The JCOG 0110 outcomes have provided clear insight, but questions concerning tumors involving the greater curvature remain.
The theoretical basis of splenectomy for removal of No. 10 LNs has been previously explored using lymphangiography. Maruyama et al. demonstrated that lymphatic flow progressed from the upper greater curvature to the splenic hilum, finally extending around the celiac trunk along the splenic artery [20]. Additionally, splenectomy is expected to have an effect by en-bloc resection of neighboring adipose tissue adjacent to the primary lesion, such as the splenogastric ligament. Based on such viewpoints, many researchers began to debate this issue by dividing their patient populations into those with cancer that did and did not involve the greater curvature. As expected, the incidence of No. 10 metastasis was higher in the Gre group in the current study, which is also consistent with previous reports [4, 6, 11, 12]. Moreover, the prognosis in the Gre group was significantly worse with more advanced stages than in the non-Gre group. This might indicate the aggressive oncological behavior of this subpopulation, although the difference probably also involved the fact that the Gre group included tumors involving the entire gastric wall circumference, such as Borrmann type 4. The overall prognosis was much worse in patients with than without No. 10 metastasis. As others have suggested, this indicates that metastasis to No. 10 is a pivotal predictor of a poor prognosis. Interestingly, only a small difference in the survival rate between patients with and without No. 10 metastasis was noted in the Gre group. This result is consistent with those reported by Ikeguchi et al. [21] and Aoyagi et al. [22]. Because of the retrospective nature of our study, we cannot determine whether splenectomy increased the survival rate. However, we believe that the effectiveness of splenectomy can be estimated using the index calculated in this study.
Although this index is not an absolute parameter, the benefits of LN dissection among various LN stations can be estimated. Indeed, the index of No. 10 in the Gre group (5.6) was more than twice that in the non-Gre group (2.0). This index in the Gre group was smaller than that of No. 1 or 3 (perigastric on lesser curvature) but almost equal to that of No. 4s (perigastric on upper greater curvature), No. 7 (trunk of left gastric artery), and No. 11 (along splenic artery). Thus, No. 10 LN dissection might have contributed to survival similarly to dissection of other stations in this subpopulation of patients. In previous reports, Nashimoto et al. [23] and Kunisaki et al. [6] reported the index of No. 10 as 4.2 and 2.4, respectively, although they evaluated all patients regardless of tumor location.
Local control by splenectomy may provide a limited oncological effect because this kind of cancer potentially exhibits very aggressive oncological behavior. Clinically, it is important to estimate which patient subpopulation is likely to benefit from splenectomy. Although the number of patient subsets was small in the current study, the index analysis indicated that relatively young patients and those with no serosal infiltration or with Borrmann type 4 tumors may benefit from splenectomy when the tumor involves the greater curvature. If serosal exposure exists, the prognosis may be controlled by peritoneal recurrence; therefore, local control by splenectomy may have little impact. Multidisciplinary therapy including perioperative chemotherapy should be considered in such cases. Borrmann type 4 is generally thought to be a poor prognostic indicator; therefore, why the index in this subgroup was relatively high remains unclear. In our series, around 60 % of type 4 tumors with positive No. 10 LNs were within T3, which might have affected this result. However, interestingly, Kosuga et al. [11] also reported that the index was relatively high in the subgroup of patients with Borrmann type 4 tumors (12.9). Some researchers have insisted that curative resection would lead to a relatively favorable prognosis despite the presence of a type 4 tumor [24, 25], particularly when serosal exposure is absent [24]. Further investigation of more patients is required to resolve this issue.
Many authors have stated that high morbidity and mortality rates are a disadvantage of splenectomy. Although the calculations of morbidity differ among various papers, previous publications have reported complication rates of 20–45 % and mortality rates of 3–12 % [5, 7, 26, 27]. JCOG 0110 trial also showed significantly higher morbidity among patients undergoing splenectomy than spleen preservation (30.3 vs. 16.1 %, respectively) [28]. In the present study, the morbidity rate in patients with grade III and higher was almost identical (26.4 %) to that in other reports, but the mortality rate was low (0.47 %). This may be because our institution is one of the highest-volume cancer centers and routinely manages postoperative complications. The potential risks of splenectomy should always be sufficiently explained to patients, especially in institutions with less experience.
Our study has several limitations. This was a retrospective study performed at a single institution, and we did not compare splenectomy and non-splenectomy. Our results suggest that a subpopulation of patients may benefit from splenectomy, but this should be further investigated in a randomized study. We should also consider whether spleen-preserving splenic hilar dissection may replace splenectomy.
In conclusion, the present study confirmed that splenectomy should be omitted in patients with tumors not involving the greater curvature, but that it may have a survival benefit in patients with tumors involving the greater curvature, especially in relatively young patients and those without serosal exposure.
References
Japanese Gastric Cancer Association (2010) Japanese classification of gastric carcinoma, 14th edn. Kanehara, Tokyo
Japanese Gastric Cancer Association (2010) Gastric cancer treatment guideline 2010, 3rd edn. Kanehara, Tokyo
Okajima K, Isozaki H (1995) Splenectomy for treatment of gastric cancer: Japanese experience. World J Surg 19:537–540. doi:10.1007/BF00294715
Monig SP, Collet PH, Baldus SE et al (2001) Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol 76:89–92
Brady MS, Rogatko A, Dent LL et al (1991) Effect of splenectomy on morbidity and survival following curative gastrectomy for carcinoma. Arch Surg 126:359–364
Kunisaki C, Makino H, Suwa H et al (2007) Impact of splenectomy in patients with gastric adenocarcinoma of the cardia. J Gastrointest Surg 11:1039–1044
Kwon SJ, Members of the Korean Gastric Cancer Study Group (1997) Prognostic impact of splenectomy on gastric cancer: results of the Korean Gastric Cancer Study Group. World J Surg 21:837–844. doi:10.1007/s002689900314
Maehara Y, Moriguchi S, Yoshida M et al (1991) Splenectomy does not correlate with length of survival in patients undergoing curative total gastrectomy for gastric cancer. Cancer 67:3006–3009
Bonenkamp JJ, Hermans J, Sasako M et al (1999) Extended lymph-node dissection for gastric cancer. N Engl J Med 340:908–914
Cuschieri A, Weeden S, Fielding J (1999) Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 79(9–10):1522–1530
Kosuga T, Ichikawa D, Okamoto K et al (2011) Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer 14:172–177
Sasada S, Ninomiya M, Nishizaki M et al (2009) Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res 29:3347–3352
Sano T, Yamamoto S, Sasako M (2002) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol 32:363–364
Sano T, Sasako M, Mizusawa J, et al (2015) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma (JCOG0110): final survival analysis. Jpn J Clin Oncol 33 (suppl 3; abstr 103)
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Dindo D, Demartines N, Clavien P (2004) Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of survey. Ann Surg 240:205–213
Sasako M, McCulloch P, Kinoshita T et al (1995) New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 82:346–351
Yu W, Choi GS, Chung HY (2006) Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 93:559–563
Zhu G, Sun Z, Wang Z et al (2012) Splenic hilar lymph node metastasis independently predicts poor survival for patients with gastric cancers in the upper and/or the middle third of the stomac. J Surg Oncol 105:786–792
Maruyama K, Okabayashi K, Kinoshita K (1987) Progress in gastric surgery in Japan and its limits of radicality. World J Surg 11:418–425. doi:10.1007/BF01655804
Ikeguchi M, Kaibara N (2004) Lymph node metastasis is at the splenic hilum in proximal gastric cancer. Am Surg 70:645–648
Aoyagi K, Kouhuji K, Miyagi M et al (2010) Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol 27:81–86
Nashimoto A, Yabusaki H, Matsui A (2012) The significance of splenectomy for advanced proximal gastric cancer. Int J Surg Oncol. doi:10.1155/2012/301530
Yoshikawa T, Tsuburaya A, Kobayashi O et al (2001) Should scirrhous gastric carcinoma be treated surgically? Clinical experiences with 233 cases and a restrospective analysis of prognosticators. Hepatogastroenterology 48:1509–1512
Kim DY, Kim HR, Kim YJ et al (2002) Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg 72:739–742
Griffith JP, Sue-Ling HM, Martin I et al (1995) Preservation of the spleen improves survival after radial surgery for gastric cancer. Gut 36:684–690
Bartlett Edmund K, Robert E et al (2014) Morbidity and mortality after total gastrectomy for gastric malignancy using the American College of Surgeons National Surgical Quality Improvement Program database. Surgery 156:298–304
Sano T, Sasako M, Shibata T, et al. (2010) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma (JCOG0110): analyzes of operative morbidity, operation time, and blood loss. J Clin Oncol 28(15s) (suppl; abstr 4020)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no commercial interest or financial support to disclose in the subject of this study.
Rights and permissions
About this article
Cite this article
Watanabe, M., Kinoshita, T., Enomoto, N. et al. Clinical Significance of Splenic Hilar Dissection with Splenectomy in Advanced Proximal Gastric Cancer: An Analysis at a Single Institution in Japan. World J Surg 40, 1165–1171 (2016). https://doi.org/10.1007/s00268-015-3362-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3362-4