Abstract
Background
Randomized clinical trials have demonstrated the limited efficacy of prophylactic drains following hepatic resection. However, many surgeons still insist on using prophylactic drains. This study was designed to identify patients who require prophylactic drains to manage or monitor postoperative complications after hepatic resection.
Methods
Data were retrospectively collected from 316 patients who underwent hepatic resection and received a prophylactic drain. The patients were divided into two groups according to whether the drain was used to manage or monitor the following postoperative complications: bile leakage (prophylactic drains were used to monitor and treat bile leakage) and postoperative hemorrhage (the drainage fluid was macroscopically bloody and required drain fluid blood counts and monitoring to assess the need for transfusion or reoperation). The results were then validated in a separate cohort of 101 patients.
Results
In 25/316 patients (7.9 %), the prophylactic drains were clinically effective, being used to manage bile leakage in 18 patients and hemorrhage in 8. Intraoperative bile leakage (P = 0.021) and long operation time (≥360 min) (P = 0.017) were independent predictors of bile leakage. Intraoperative blood loss (≥650 ml) (P = 0.0009) was an independent predictor of hemorrhage. In the subsequent 101 patients, prophylactic drains were clinically effective in patients with one of these predictors with sensitivity, specificity, and false-negative rates of 88.9, 62.0, and 1.7 %, respectively.
Conclusion
A prophylactic drain should be considered following hepatic resection for patients with intraoperative bile leakage, operation time of ≥360 min, or blood loss of ≥650 ml.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of recent advances in surgical techniques, hepatic resection is being performed with increasing frequency, and the mortality and morbidity rates are steadily decreasing [1, 2]. Several recent randomized clinical trials (RCTs) have revealed that prophylactic drains are unnecessary after elective liver resection [3, 4]. Moreover, prophylactic drains may increase the risk of surgical site infection (SSI) [3, 5]. Nevertheless, many surgeons continue to insert prophylactic drains because they are thought to facilitate early detection and treatment of complications such as bile leakage or hemorrhage [6, 7].
Considering that the effectiveness of prophylactic drains may depend on the individual patient’s characteristics, it may be prudent to limit their use to patients who are likely to benefit in terms of the management and treatment of postoperative complications. Some reports have proposed criteria for early drain removal based on the drainage fluid bilirubin level and fluid volume measured on postoperative day (POD) 3 [5, 7]. Other studies have revealed risk factors for additional postoperative procedures based on the hepatectomy method, intraoperative bile leakage, and intraoperative blood loss [8, 9]. To our knowledge, however, no prior studies have attempted to identify criteria for using prophylactic drains in individual patients. Therefore, the aim of the present study was to identify possible clinical predictors of effective use of a prophylactic drain to detect and manage postoperative complications following hepatic resection.
Patients and methods
Patients
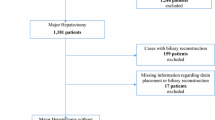
Two analyses were conducted in this study. The first analysis retrospectively assessed the characteristics of 370 patients whose prophylactic drains were clinically effective in the short-term postoperative period after elective hepatic resection, including 69 laparoscopic resections, at the Department of Surgery and Science, Kyushu University Hospital from January 2009 to December 2013. Forty-eight patients who underwent concomitant procedures on other organs, including bile duct reconstruction or gastrointestinal resection, were excluded from this study. Overall, 316/322 patients (98.1 %) received prophylactic drains and were enrolled in this study. The reasons for hepatic resection in these 316 patients included hepatocellular carcinoma (n = 212, 67 %), other liver carcinomas (n = 27, 9 %), liver metastases (n = 60, 19 %), benign tumors (n = 11, 3 %), and liver cysts (n = 6, 2 %). The operative procedures were as follows: lobectomy (n = 45, 14 %) or resection of more than one segment (n = 6, 2 %), segmentectomy (n = 60, 19 %), subsegmentectomy (n = 47, 15 %), and partial hepatectomy (n = 158, 50 %). One prophylactic drain was routinely inserted near the cut surface of the liver at the end of surgery. The second analysis was performed to validate the reliability of the results obtained from the first analysis and included data from a separate cohort of 101 patients who underwent hepatic resection from January 2014 to March 2015.
Study design
We divided the 316 patients into 2 groups according to whether the drain was used to manage or monitor postoperative complications. Predictors of the effectiveness of prophylactic drains were identified using multivariate analysis of clinical factors. The accuracy of the identified predictors was assessed in the subsequent cohort of 101 patients.
Surgical procedures and patient management
Patients were carefully selected for major hepatic resection based on volumetric analysis of the remnant liver to prevent postoperative liver failure [10–12]. The type of hepatic resection was determined according to the preoperative indocyanine green (ICG) retention rate at 15 min [13]. Parenchymal dissection of the liver was performed using a Cavitron Ultrasonic Surgical Aspirator system (CUSA system, Valleylab Inc., Boulder, CO, USA) and a VIO system (VIO 300D; ERBE Elektromedizin, Tübingen, Germany) containing a soft-coagulation mode tip (SOFT COAG) and a bipolar clamp (BiClamp) for laparoscopic resection [14, 15]. Intraoperative blood flow control was achieved with the Pringle maneuver (15-min occlusion and 5-min release). Possible bile leakage was tested using ICG solution whenever possible [16] (n = 121, 38.3 %). We defined intraoperative bile leakage as a positive intraoperative bile leakage test result or the presence of bile on the cut surface of the liver during surgery. If bile leakage was detected, the leakage point was repaired by Z-sutures using 6–0 polydioxanone (PDSII; Johnson & Johnson, Tokyo, Japan). An absorbable polyglactin acid sheet mesh (Neoveil, Gunze, Tokyo, Japan) and fibrin glue (Bolheal; Kaketsuken, Kumamoto, Japan or Beriplast; Nycomed, Zurich, Switzerland) were routinely applied to the cut surface of the liver. Anticoagulant drugs, such as nafamostat mesilate [17], were administered perioperatively to prevent postoperative disseminated intravascular coagulation. The patients were also preoperatively administered 500 mg of methylprednisolone [18].
Drain insertion and management
A 15-Fr closed suction drain (J-Vac drain; Johnson & Johnson, Somerville, NJ, USA) was inserted near the cut surface of the liver at the end of surgery. Fluid was aspirated from the drain daily to measure the total bilirubin concentration [19]. The prophylactic drains were usually removed by POD 3 unless the drainage fluid was grossly bloody, exceeded 100 ml/day, or did not meet the bile leakage criteria of the International Study Group of Liver Surgery (ISGLS) [19]. In these cases, the drainage tube was retained until the fluid turned clear, bile leakage disappeared [19], or the drainage volume decreased to <100 ml/day. If the drainage fluid volume of serous ascites was the only reason to retain the drainage tube, the wound around the drain site was sutured after drain removal.
Definitions of clinical effectiveness of prophylactic drains
A prophylactic drain was considered clinically effective if the drainage fluid color or volume contributed to the guidance or treatment of the following postoperative complications: bile leakage (n = 18) or postoperative hemorrhage (n = 8). Routine abdominal X-rays were obtained just after the operation and the next day to avoid dislocation of the prophylactic drains; therefore, we consider that all prophylactic drains actually worked to monitor the postoperative complications. For bile leakage, the prophylactic drain was deemed effective if it was used to monitor the severity of bile leakage. Bile leakage was defined according to the criteria of the ISGLS as a drain fluid-to-serum total bilirubin concentration ratio of ≥3.0 on or after POD 3. Beyond POD 3, we continued to monitor the drain if the bilirubin ratio remained ≥3.0. Postoperative hemorrhage was defined as drainage fluid that was macroscopically bloody and required multiple blood tests or a drainage fluid blood count to check for progressive anemia. The severity of postoperative hemorrhage was monitored to assess the need for transfusion or reoperation.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. In univariate analyses, continuous variables were compared using Student’s t test and categorical variables were compared using the χ 2 test or Fisher’s exact test. Variables with a P value of <0.05 in the univariate analysis were included in a stepwise logistic regression analysis to identify independent predictors of bile leakage and postoperative hemorrhage. In all analyses, P < 0.05 was considered statistically significant. Estimation of the cutoff values of operation time and intraoperative blood loss for predicting bile leakage and postoperative hemorrhage, respectively, was performed by calculating the areas under the receiver operating characteristic (ROC) curves. The ROC curve is a plot of sensitivity versus 1—specificity for all possible cutoff values. The optimal cutoff values used were selected based on the sensitivity, specificity, positive predictive value, and negative predictive value. All analyses were performed using JMP Version 9.0.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Characteristics of patients with clinically effective prophylactic drains
The patients were divided into two groups: patients whose drain was clinically effective (n = 25, 7.9 %) and patients whose drain was not clinically effective (n = 291, 92.1 %). In one patient, the drain was effective in terms of detecting early postoperative hemorrhage from POD 0 to POD 1 and bile leakage from POD 8 to POD 31. Therefore, the drains were effective in 26 patients (bile leakage in 18 patients and postoperative hemorrhage in 8 patients). Ten patients with bile leakage needed no additional radiological or surgical intervention. Additional treatment with endoscopic retrograde cholangiopancreatography, exchange of the drainage tube, establishment of secondary percutaneous drainage, and surgery were performed in three, three, one, and one patient, respectively. Of the eight patients with postoperative hemorrhage, five were managed with fresh frozen plasma; one of these five required relaparotomy.
Predictors of the effectiveness of prophylactic drains for managing bile leakage
Clinical factors related to the effectiveness of prophylactic drains for managing bile leakage, including patient characteristics, surgical factors, and tumor-related factors, were compared between the patients without and with effective drains (Table 1). In the univariate analyses, patients without and with effective drains exhibited statistically significant differences in body mass index (22.9 vs. 24.7 kg/m2, respectively; P = 0.030), operation time (317 vs. 419 min, respectively; P = 0.0005), and intraoperative bile leakage (20.1 vs. 55.6 %, respectively; P = 0.0004). Multivariate logistic regression revealed that operation time (P = 0.021) and intraoperative bile leakage (P = 0.017) were significantly associated with the effectiveness of prophylactic drains for managing bile leakage. Figure 1a shows the ROC curve of operation time. The optimal cutoff value was 385 min (sensitivity, 0.61; specificity, 0.75). Similar sensitivity and specificity values (0.67 and 0.68, respectively) were obtained when we used a cutoff value of 360 min, which is simpler in clinical use. Therefore, we defined the cutoff value of operation time as 360 min for predicting the effectiveness of prophylactic drains.
a Receiver operating characteristic curves using operation time as a predictor of the effectiveness of prophylactic drains in the management of bile leakage. b Receiver operating characteristic curves using operation time as a predictor of the effectiveness of prophylactic drains in the management of postoperative hemorrhage. AUC area under the curve
Predictors of the effectiveness of prophylactic drains for managing postoperative hemorrhage
In the univariate analyses to identify factors related to the effectiveness of prophylactic drains for managing postoperative hemorrhage, patients without and with effective prophylactic drains exhibited significant differences in operation time (321 vs. 429 min, respectively; P = 0.018), intraoperative blood loss (511 vs. 1560 ml, respectively; P < 0.001), intraoperative blood transfusion (11.8 vs. 33.3 %, respectively; P = 0.028), and the duration of the Pringle maneuver (48 vs. 85 min, respectively; P = 0.021) (Table 2). In the multivariate analysis, the only independent predictor was intraoperative blood loss (P = 0.008). Figure 1b shows the ROC curve of blood loss. Because the optimal cutoff value for blood loss was 650 ml (sensitivity, 0.78; specificity, 0.75), we used this as the cutoff value for predicting the effectiveness of prophylactic drains.
Validation of the predictors of the effectiveness of prophylactic drains
The predictors of the effectiveness of prophylactic drains (intraoperative bile leakage, operation time of ≥360 min, and blood loss of ≥650 ml) were validated in the second analysis using a separate cohort of 101 patients. The sensitivity was 88.9 %, and the specificity was 62.0 %. Of 58 patients who had none of these predictors, 1 (1.7 %) had a clinically effective prophylactic drain resulting in a negative predictive value of 98.3 %. Of 43 patients with any of these predictors, 8 (18.6 %) had clinically effective prophylactic drains, resulting in a positive predictive value of 18.6 %. Therefore, the accuracy of our predictors was 64.4 %.
Discussion
The present study showed that prophylactic drains were effective in 25 patients (7.9 %, 26 cases), including 18 patients with bile leakage (5.7 %) and 8 patients with postoperative hemorrhage (2.5 %), similar to the results of other studies [6, 7]. Refractory ascites is another important problem after hepatic resection, but in this study we focused more on the early effectiveness, not long-term effectiveness. When we analyzed predictors of the effectiveness of prophylactic drains for refractory ascites, independent factors were a low serum albumin level (<3.9 mg/dl) (P = 0.010) and long operation time (≥360 min) (P = 0.023), the latter of which is also one of our criteria.
Less than half of the patients (43/101) met our predictors of prophylactic drain insertion. The others had none of the predictors, and we could have skipped inserting a prophylactic drain in these patients. Notably, our predictors of effective prophylactic drain insertion included all of the patients with major complications (n = 5, 5.0 %) of Clavien grade IIIa or greater [20], which suggests that these predictors should allow us to identify patients who require drain insertion for safety concerns.
Several reports have proposed criteria for selective drain placement based on the need for additional postoperative interventions [8, 9]. Our criteria differ from theirs because we evaluated prophylactic drains not only as therapeutic tools, but also as diagnostic tools. Hirokawa et al. [8] reported the following predictors of effective prophylactic drain insertion: extended hepatic resection with portal vein thrombus, use of a high-risk hepatectomy procedure (e.g., central bisegmentectomy or anterior segmentectomy exposing the major Glisson’s sheath), repeated hepatic resection, and intraoperative bile leakage. When we applied these criteria to our 101 patients in the second analysis of the present study, the sensitivity was 66.7 % (6/9) and the specificity was 50.0 % (46/92). Sensitivity was lower than our criteria (88.9 %), which could induce omitting patients who should have prophylactic drains. Ishizawa et al. [9]. Reported the following criteria for prophylactic drain insertion: blood loss of >400 ml, preoperative chemotherapy, intraoperative bile leakage, bilioenteric anastomosis, and increased risk of postoperative bleeding. When we applied these criteria to our 101 patients in the second analysis, the sensitivity was 88.9 % (8/9) and the specificity was 38.0 % (35/92). These criteria only applied to 35.6 % of the patients (35/101) to the no-drain group. Because Ishizawa et al. [9] focused on laparoscopic hepatic resection, the criteria might not be appropriate for patients undergoing other surgical procedures.
In our series, postoperative complications classified as Clavien grade II or greater occurred in 28.6 % of patients (90/316), and reoperation was necessary in 0.9 % of patients (3/316). In the present study, there were no in-hospital mortalities. In earlier studies with a no-drain policy after hepatic resection, reoperation was necessary in 1.9 % of patients (1/52 cases) [21] and the in-hospital mortality rate was 1.7 % (1/60) in one study [21] and 1.9 % (1/52) in another study [3]. Prophylactic drains could have allowed the early detection and treatment of postoperative complications, prevented in-hospital mortality, and reduced the need for reoperation. By contrast, the rate of complications in patients with a prophylactic drain was similar to that in patients without prophylactic drains (28.6 %) in another study [8]. SSI, which were defined using standardized surveillance criteria [22], occurred in 11.4 % of patients (36/316), which is similar to the rate in patients with no drains (13.5 %) [21]. Prophylactic drains can be safely placed without increasing the risk of SSI through optimal drain management [7].
Although numerous studies have recommended a no-drain policy [3, 8, 9, 21], several patients in our series benefited from a prophylactic drain without requiring additional invasive procedures. Selective placement of a drain to manage or prevent postoperative complications in high-risk patients appears to be reasonable, and the criteria outlined in our study should help surgeons to select patients likely to benefit from a prophylactic drain.
The main limitation of this study is the retrospective data collection and analysis. Additional reports from other centers are necessary to help generalize our findings. Our criteria should play an important role in helping future RCTs to elucidate the clinical role of prophylactic drains in a relatively safe manner.
In conclusion, prophylactic drain insertion after hepatic resection is clinically effective in terms of the management of bile leakage and postoperative hemorrhage. Predictors of effective prophylactic drains were the presence of intraoperative bile leakage, operation time of ≥360 min, and blood loss of ≥650 ml.
References
Yamashita Y, Tsuijita E, Takeishi K et al (2014) Trends in surgical results of hepatic resection for hepatocellular carcinoma: 1000 consecutive cases over 20 years in a single institution. Am J Surg 207:890–896
Taketomi A, Kitagawa D, Itoh S et al (2007) Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute’s experience with 625 patients. J Am Coll Surg 204:580–587
Sun HC, Qin LX, Lu L et al (2006) Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg 93:422–426
Belghiti J, Kabbej M, Sauvanet A et al (1993) Drainage after elective hepatic resection. A randomized trial. Ann Surg 218:748–753
Tanaka K, Kumamoto T, Nojiri K et al (2013) The effectiveness and appropriate management of abdominal drains in patients undergoing elective liver resection: a retrospective analysis and prospective case series. Surg Today 43:372–380
Torzilli G, Olivari N, Del Fabbro D et al (2005) Bilirubin level fluctuation in drain discharge after hepatectomies justifies long-term drain maintenance. Hepatogastroenterology 52:1206–1210
Yamazaki S, Takayama T, Moriguchi M et al (2012) Criteria for drain removal following liver resection. Br J Surg 99:1584–1590
Hirokawa F, Hayashi M, Miyamoto Y et al (2011) Re-evaluation of the necessity of prophylactic drainage after liver resection. Am Surg 77:539–544
Ishizawa T, Zuker NB, Conrad C et al (2014) Using a ‘no drain’ policy in 342 laparoscopic hepatectomies: which factors predict failure? HPB (Oxford) 16:494–499
Shirabe K, Shimada M, Gion T et al (1999) Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 188:304–309
Kayashima H, Shirabe K, Matono R et al (2014) Three-dimensional computed tomography analysis of variations in the middle hepatic vein tributaries: proposed new classification. Surg Today 44(11):2077–2085
Yonemura Y, Taketomi A, Soejima Y et al (2005) Validity of preoperative volumetric analysis of congestion volume in living donor liver transplantation using three-dimensional computed tomography. Liver Transpl 11:1556–1562
Yamashita Y, Taketomi A, Itoh S et al (2007) Longterm favorable results of limited hepatic resections for patients with hepatocellular carcinoma: 20 years of experience. J Am Coll Surg 205:19–26
Ikeda T, Toshima T, Harimoto N et al (2014) Laparoscopic liver resection in the semiprone position for tumors in the anterosuperior and posterior segments, using a novel dual-handling technique and bipolar irrigation system. Surg Endosc 28:2484–2492
Itoh S, Fukuzawa K, Shitomi Y et al (2012) Impact of the VIO system in hepatic resection for patients with hepatocellular carcinoma. Surg Today 42:1176–1182
Yamashita Y, Hamatsu T, Rikimaru T et al (2001) Bile leakage after hepatic resection. Ann Surg 233:45–50
Shimada M, Matsumata T, Shirabe K et al (1994) Effect of nafamostat mesilate on coagulation and fibrinolysis in hepatic resection. J Am Coll Surg 178:498–502
Yamashita Y, Shimada M, Hamatsu T et al (2001) Effects of preoperative steroid administration on surgical stress in hepatic resection: prospective randomized trial. Arch Surg 136:328–333
Koch M, Garden OJ, Padbury R et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149:680–688
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Liu CL, Fan ST, Lo CM et al (2004) Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg 239:194–201
Mangram AJ, Horan TC, Pearson ML et al (1999) Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27:97–132 (quiz 3–4; discussion 96)
Conflict of interest
The authors declare that they have no conflicts of interest or financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bekki, Y., Yamashita, Yi., Itoh, S. et al. Predictors of the Effectiveness of Prophylactic Drains After Hepatic Resection. World J Surg 39, 2543–2549 (2015). https://doi.org/10.1007/s00268-015-3116-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3116-3